Obtaining FDA Certification is a critical milestone for any product, especially when it comes to health and wellness devices. However, the certification process can be further strengthened and validated by a Clinical Test Report. These reports provide the scientific backing necessary to prove that a product meets safety, efficacy, and performance standards, making it more reliable in the eyes of both regulatory bodies and consumers.
An FDA Certification is a mark of regulatory approval, but it doesn’t always provide the full scope of evidence needed for consumer trust. A Clinical Test Report goes a step further by providing documented, real-world results, demonstrating how the product performs in actual use. This adds credibility to the product’s claims and ensures that the product delivers what it promises.
While FDA Certification ensures compliance with federal regulations, the addition of a Clinical Test Report verifies that the product adheres to strict health standards, including safety and efficacy. This combination is particularly important for medical devices or consumer health products, as it assures that the product is safe for long-term use and effective in achieving its intended purpose.
For products that require FDA Certification, especially in the healthcare and wellness industry, consumers often look for more than just a seal of approval. A Clinical Test Report offers a transparent overview of testing procedures, outcomes, and data, which can increase consumer confidence in the product’s reliability and effectiveness. This transparency leads to a stronger customer base and positive brand reputation.
Having both FDA Certification and a supporting Clinical Test Report makes it easier for companies to enter global markets. Countries with stringent regulatory requirements, such as those in the European Union or Japan, often require not only FDA Certification but also verified clinical data. A Clinical Test Report can help smooth the approval process in these regions and open doors for international sales and distribution.
In the event of product claims or issues, a Clinical Test Report can serve as critical evidence. This documentation can provide legal protection against product liability claims, offering peace of mind to manufacturers and business partners.
For manufacturers working with OEMs or distributors, a combination of FDA Certification and Clinical Test Report strengthens the business relationship. Partners are more likely to trust a product that is backed by clinical data. This enhances collaboration and helps ensure long-term partnerships in the marketplace.
In the highly competitive landscape of health and wellness products, FDA Certification is essential, but it’s the inclusion of a Clinical Test Report that truly solidifies a product’s place in the market. This combination ensures that the product not only meets regulatory requirements but also provides documented proof of its safety. Contact us


Are the Integrated Invisible Buttons of the Electric Toothbrush Good or Bad?

Reasons to enter the oral irrigators industry in 2024
.jpg)
Sonic Toothbrush Manufacturer China – OEM & Bulk Production

How to Compare Electric Toothbrush Suppliers When Selecting a Philips Sonicare Supplier Wholesale?

Can an Electric Toothbrush Designer Drive Smart Toothbrush Innovation with AI Features?
Solving Electric Toothbrush Roll & Fall: Product Development Strategies for OEMs

How Does High Quantum Yield Optimize the Performance of a Smart Timer Whitening Kit?
.jpg)
Why Dentist Consultations Mitigate Home Treatment Risks?

Why Choose Our Electric Toothbrush ODM as Your Premier Teeth Whitening Kit Supplier?
.jpg)
Why Optimize Light Diffusion Lens Design Based on Peroxide Decomposition Rate?
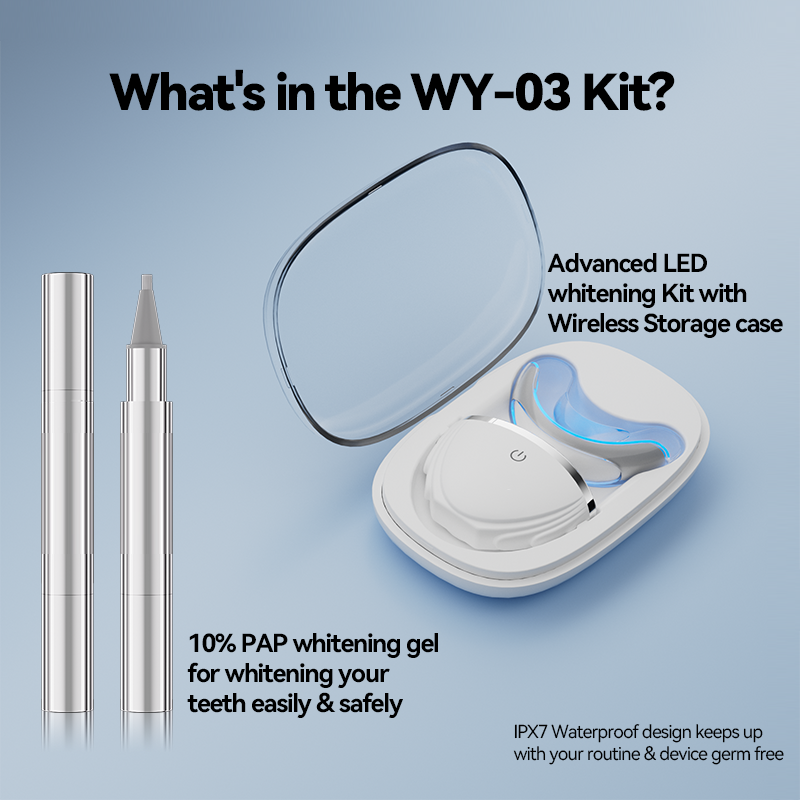
What Gives Some Teeth Whitening Devices Competitive advantage?
.jpg)
Confused by dental insurance? Which coverage plans include implants?
.jpg)
High-Frequency Toothbrush Wholesale for Global B2B Distribution

Why Does the Oral Irrigator Make Abnormal Noise After Being Used for a Period of Time?
.jpg)
Tooth Chipping Alongside Root Darkening – Irreversible?

Should Nozzle Material Always Be Medical-grade Material for Safety?