water flosser pressure calibration
For professional oral care brands, product performance is never random. Instead, every technical parameter should follow controlled engineering logic. Pressure output is one of the most critical performance indicators in a water flosser, because it directly affects user comfort, gum safety, and cleaning efficiency. Therefore, structured control of pressure settings becomes essential in OEM manufacturing.
Consumers expect strong cleaning without discomfort. However, if water pressure fluctuates, the device may feel unpredictable or even irritating. Inconsistent output often results from pump variation, nozzle resistance, internal tube friction, and manufacturing tolerance. When calibration programs are weak, customer complaints and warranty claims usually increase. Consequently, brands need factory partners who understand validation testing.
Moreover, stable pressure helps your marketing claims remain credible. Retail partners and distributors prefer measurable performance standards rather than subjective statements. This is why mature manufacturers design test benches to measure pressure curves across working modes.
Besides hardware engineering, calibration methods determine final consistency. Responsible suppliers set allowable tolerance windows and verify samples from each batch. Additionally, they monitor both peak output and long-term stability as components age. Because water minerals and internal vibration may influence performance, life-cycle testing becomes necessary.
In the middle of your project documentation, the factory should demonstrate one clear instance of water flosser pressure calibration using real test procedures. Data logging systems allow engineers to track deviations and adjust assembly alignment. As a result, your finished devices stay predictable in different environments.
Although water flossers are not always classified as medical equipment, regulators and retailers still expect documentation discipline. Therefore, keeping calibration records supports safety audits and market entry reviews. Furthermore, technical transparency improves trust between brand owners and manufacturing partners.
When OEM partners share early-stage engineering knowledge, you can optimize device design before full tooling investment. This cooperation decreases rework rates and reduces downstream risk. Additionally, your product planning team gains better visibility into cost, scrap rate, and long-term serviceability.
To explore an example of an experienced oral care manufacturer, you may review:
https://powsmart.com/products/oral-irrigator
And for broader oral hygiene guidance, industry authorities such as the American Dental Association publish useful resources:
https://www.ada.org/
Because calibration systems require investment, only structured factories can maintain them effectively. However, the payoff is significant: lower returns, stronger product reviews, and improved distributor confidence. Consequently, brands that prioritize pressure stability usually achieve higher lifetime value from each customer.
In a competitive category, engineering discipline sets you apart. OEM buyers who evaluate calibration capability as a key supplier selection factor will experience fewer operational surprises, along with smoother market launches.
.jpg)
.jpg)
.jpg)
.jpg)
Long-Term Toothbrush Supplier Partnership for Sustainable Growth
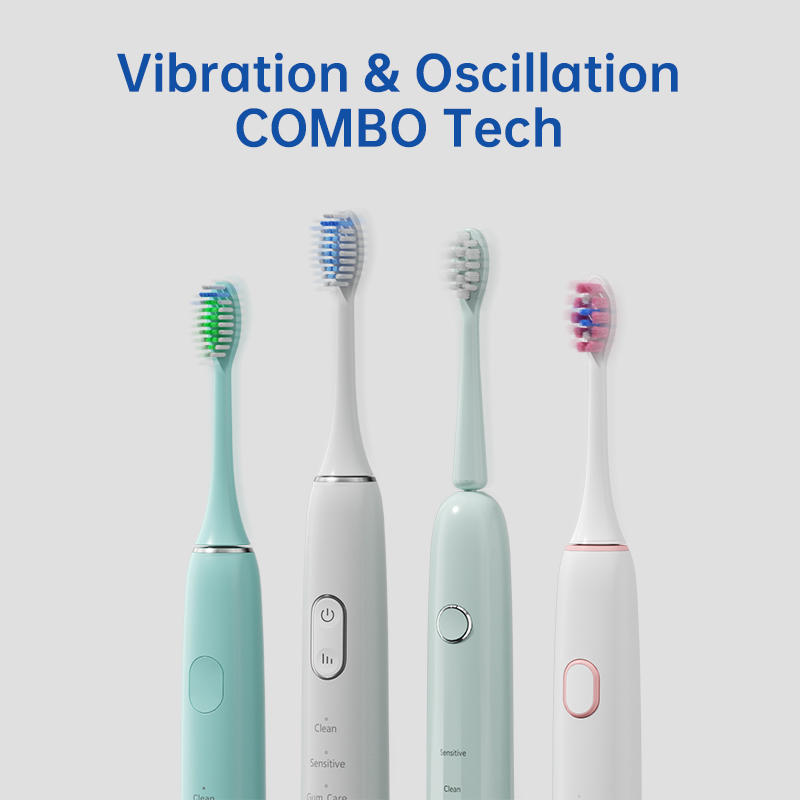
Can an Apical Irrigation Tip Utilize Hydro-kinetic Energy Transfer for Root Canal Cleaning?
.jpg)
Wholesale Electric Toothbrush Vendor Spokane Gift Shops | Affordable Supply
.jpg)
Queens toothbrush store — should it honor Long Island deals? A B2B Playbook for Seamless Cross-Borough Promotions

No Water Flow? How OEM Engineering Prevents Common Water Flosser Failures
.jpg)
Seeking dental assistant roles? Certification programs near Tacoma

Why Combine a Photobleaching Catalyst with an Optical Brightener in Advanced Whitening Formulas?

What Are the Most Important Aspects to Pay Attention to When Looking for Electric Toothbrush Suppliers in China?
.jpg)
Whitening Pen + LED Kit Supplier | Teeth Whitening Combo OEM
.jpg)
Water Flosser Gearbox Assembly & Drop Test: Ensuring Reliability and Durability
.jpg)
Biodegradable Electric Toothbrush OEM | Eco-Friendly Oral Care Manufacturing
.jpg)
Oakland toothbrush deals — can you claim a California tax-free benefit?

What Makes a Portable Water Flosser Truly User-Friendly?
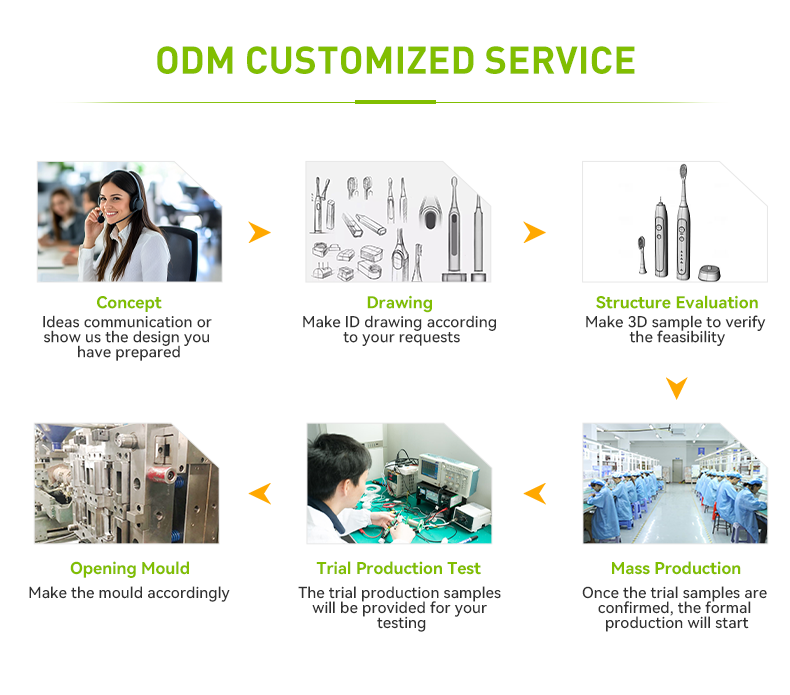
Need Brush Head Injection Molding Expertise with Certified Food-grade Bristle Material?
.jpg)
water flosser OEM factory
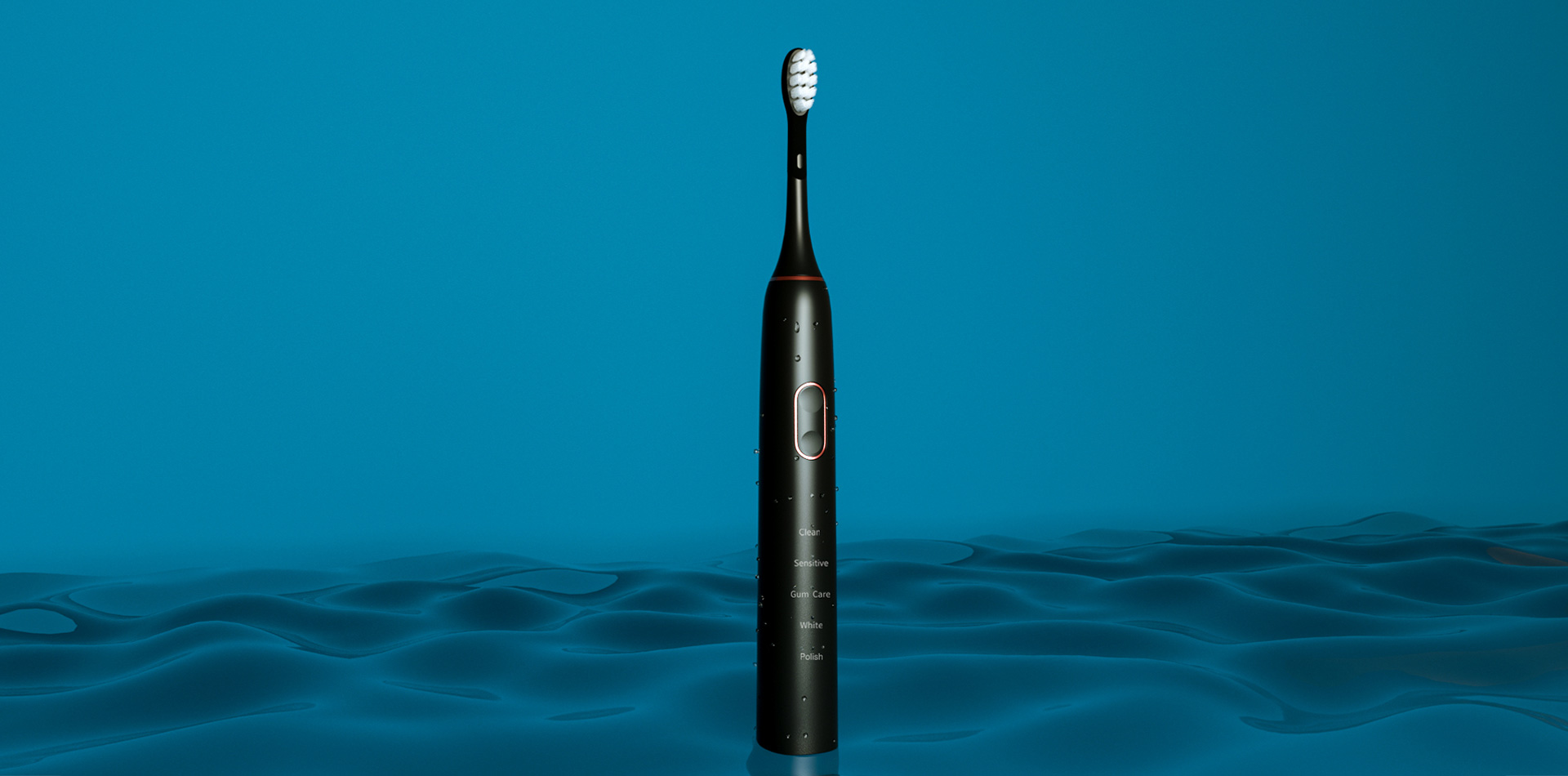
Looking for Sonic Toothbrush OEM Manufacturing and Water Flosser Private Label Services?