With consumer expectations rising, the demand for higher safety and quality in oral care devices like electric toothbrushes is stronger than ever. In this blog, Powsmart will explain how electric toothbrush manufacturers align with medical device production standards to achieve ISO 13485 electric toothbrush and deliver products trusted by both dental professionals and consumers.https://www.powsmart.com/about-powsmart/
ISO 13485 is an internationally recognized standard specifically for medical device quality management systems. While electric toothbrushes are often classified as consumer electronics, premium models, especially those used in clinical or specialized dental care, are increasingly expected to meet medical device production standards.
Compliance with ISO 13485 electric toothbrush guidelines ensures:
Safer product design and manufacturing
Consistent quality control across batches
Enhanced credibility in both consumer and professional markets
Manufacturers adopting this system stand out with superior reliability and performance.
Meeting ISO 13485 starts with establishing a comprehensive quality management system.
Key components include:
Detailed documentation at every production stage
Traceability for all components and processes
Risk assessment and corrective action plans
Continuous training for quality and production teams
By building a robust QMS, manufacturers ensure that each electric toothbrush meets strict international requirements, minimizing defects and customer complaints.
Though electric toothbrushes are not invasive devices, maintaining high levels of hygiene during production is essential—especially for models used in medical or orthodontic applications.
Professional manufacturers operate sterile production workshops to:
Minimize microbial contamination
Control environmental factors like dust, humidity, and airborne particles
Meet the cleanliness levels outlined by medical device production standards
This sterile manufacturing environment elevates product safety and helps achieve necessary oral care product certifications.
Obtaining oral care product certification under ISO 13485 involves several critical steps:
Design verification and validation to confirm product safety and effectiveness
Independent audits and factory inspections
Continuous monitoring and re-certification to ensure ongoing compliance
Manufacturers who invest in this process not only demonstrate quality but also gain access to premium distribution channels such as dental clinics, hospitals, and specialized oral care stores.
In today’s competitive market, ensuring your products meet medical device production standards is no longer optional—it’s essential. Through:
Implementing a full quality management system
Operating sterile production workshops
Achieving oral care product certification like ISO 13485
Manufacturers can guarantee that their ISO 13485 electric toothbrush products deliver superior safety, performance, and reliability.
Partner with a certified manufacturer today to elevate your brand’s reputation and meet the next generation of consumer expectations! https://www.powsmart.com/contact-us/

How to Effectively Reduce the After-Sales Return Rate of Electric Toothbrushes
Frequent Allergy Reactions to Whitening Kits: Is Cold-Light Technology to Blame?

Gingival Abrasion with Saliva Depletion – A Silent Crisis in Oral Care Design?

The Importance of Water Resistance for Teeth Whitening Devices and Anti-Saliva Corrosion Structure
Simple One Button Electric Toothbrush for Senior Citizens Pune
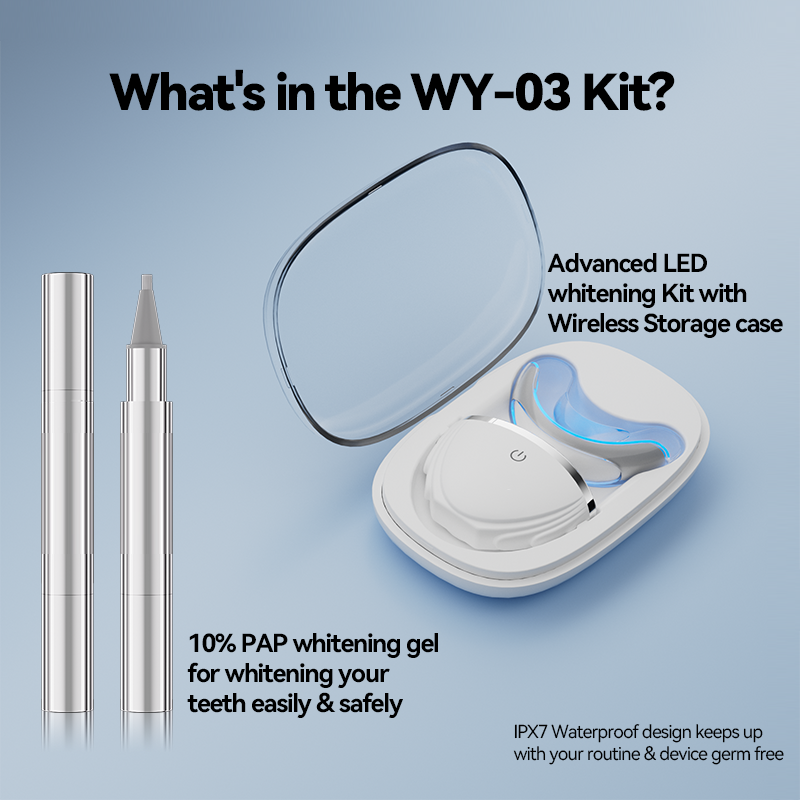
OEM of Teeth Whitening Device: A Complete Solution from LOGO Printing to Light Wave Mode Customization
How to Deal with Sudden Tooth Sensitivity carefully?
Are Pressure Sensor Errors Worsening Hygiene Mode Absence?

How Can Oral Care Product Distributors Optimize Product Details to Increase Sales?
.jpg)
Smart Electric Toothbrush Manufacturing: How to Choose the Best OEM Partner

Is a One-button toothbrush truly a Pune simple toothbrush for seniors?
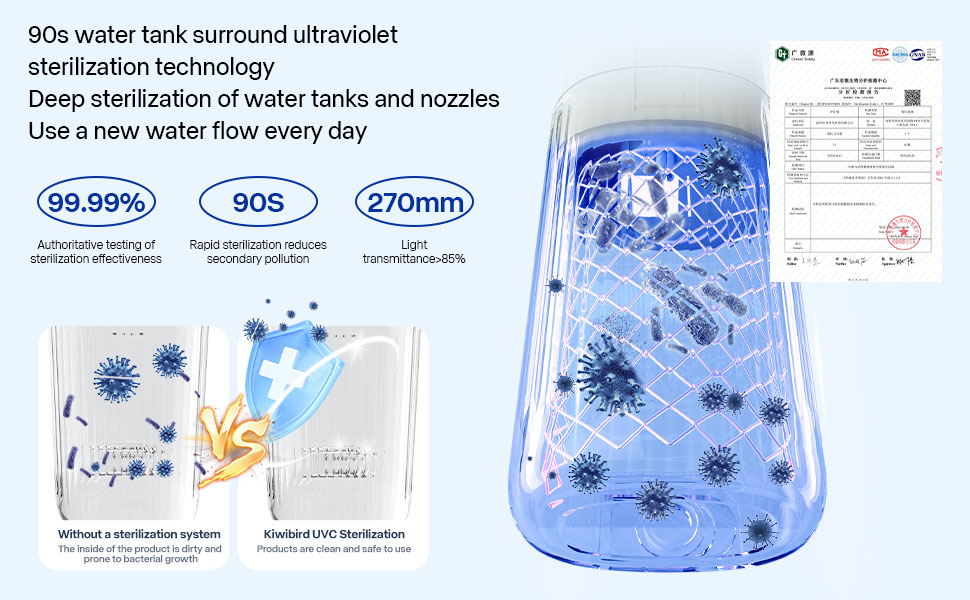
Does a Water Flosser Require Regular Maintenance? How Should It Be Cleaned?

Beginner’s Guide to Water Flosser Pressure Settings

The 3 Most Common Tests for FDA Rejection of Home Teeth Whitening Devices
.jpg)
Executive Diwali gift or Status symbol toothbrush — what truly impresses?
Benefits of Red & Blue Light Technology in Professional Teeth Whitening Kits
.jpg)
Florida Electric Toothbrush – Powsmart PTR-C8

electric toothbrush heads Deep Clean

electric toothbrush heads Ultra Soft

Customization Teeth Whitening Gel

electric toothbrush heads Charcoal Infuse-Round

Electric toothbrush heads Charcoal Infused-Diamond

electric toothbrush heads Regular Clean

Private Label Whitening Gel
whstapp
whstapp
National Toll-Free Service Hotline
+86 755 86238638