For global distributors and private label brands, understanding Toothbrush import regulations is a crucial step in building a compliant and reliable supply chain. Whether you’re importing manual or electric toothbrushes, each region enforces its own toothbrush quality standards that manufacturers and importers must meet. Failure to comply can result in shipment delays, customs rejections, or even financial penalties.
In this blog, we’ll outline six key areas every B2B buyer should review to confidently navigate international toothbrush import requirements.
Each destination market has unique Toothbrush import regulations that define what materials, labeling, and documentation are required. For instance, the U.S. Food and Drug Administration (FDA) categorizes toothbrushes as medical devices, while the European Union enforces CE marking and REACH compliance for materials safety.
Before placing bulk orders, buyers should confirm that their supplier understands and meets the necessary compliance criteria for your target market. A knowledgeable manufacturer will already be familiar with regional certification processes, ensuring smooth customs clearance.
To meet toothbrush quality standards, suppliers should conduct routine product testing for bristle firmness, handle durability, and material safety (e.g., BPA-free plastics). Industry benchmarks such as ISO 20126 or ADA guidelines serve as global references for product performance and safety.
Importers can request test reports, safety data sheets (SDS), or independent lab certifications to verify compliance. Partnering with a manufacturer that consistently meets these standards reduces the risk of shipment rejection during import inspections.
Proper labeling and documentation play a significant role in toothbrush import regulations. Commonly required documents include the Certificate of Origin, Product Safety Certificate, Material Declaration, and Packaging Compliance details.
Additionally, labeling must accurately display country of manufacture, batch codes, and usage instructions in the destination language. Manufacturers experienced in export documentation can streamline this process, minimizing the chance of customs delays or compliance disputes.
To align with toothbrush quality standards, many importing countries restrict the use of certain materials such as phthalates, heavy metals, or non-food-grade colorants. Compliance with REACH (EU), Prop 65 (California), or GB standards (China) ensures product safety and consumer trust.
When sourcing from overseas, always confirm that your manufacturer conducts raw material audits and obtains supplier declarations to validate compliance with these chemical and material restrictions.
Even with full documentation, customs authorities may perform random inspections or laboratory testing. Reliable suppliers should maintain a consistent quality record to minimize the likelihood of testing failures.
By partnering with an experienced factory that adheres to toothbrush quality standards, importers can benefit from reduced clearance times and a stronger track record of regulatory compliance.
Choosing a manufacturing partner familiar with toothbrush import regulations can simplify your entire import process. Reputable suppliers understand the regulatory expectations for multiple markets, maintain certification libraries, and proactively prepare compliant documentation.
This partnership not only protects your brand from compliance risks but also accelerates market entry. Suppliers who integrate toothbrush quality standards into every production stage ensure that your products pass both factory and border inspections seamlessly.
Successfully navigating toothbrush import regulations requires thorough preparation, supplier collaboration, and an understanding of international toothbrush quality standards. From raw material verification to labeling and export documentation, every step contributes to a compliant and efficient import process.
Working with a trusted, globally certified toothbrush manufacturer ensures that your brand remains competitive and compliant—no matter where your products are headed.
? Looking for an experienced export partner with proven compliance expertise? Contact our team to learn how we help brands meet import standards across global markets.
.jpg)
.jpg)
Electric Toothbrush Production Factory for OEM Manufacturing
.jpg)
NYC Teeth Whitening Kits | Fast, Stylish At-Home Solutions
.jpg)
E-commerce Toothbrush Supplier for Online Brands & Distributors
.jpg)
Vestibular Stimulation Triggering Head Positioning Dizziness?

Is POWSMART Ultrasonic Toothbrush’s Chemical Residue Over Limit?
.jpg)
How Dangerous Are Jet Instability and Power Surges?
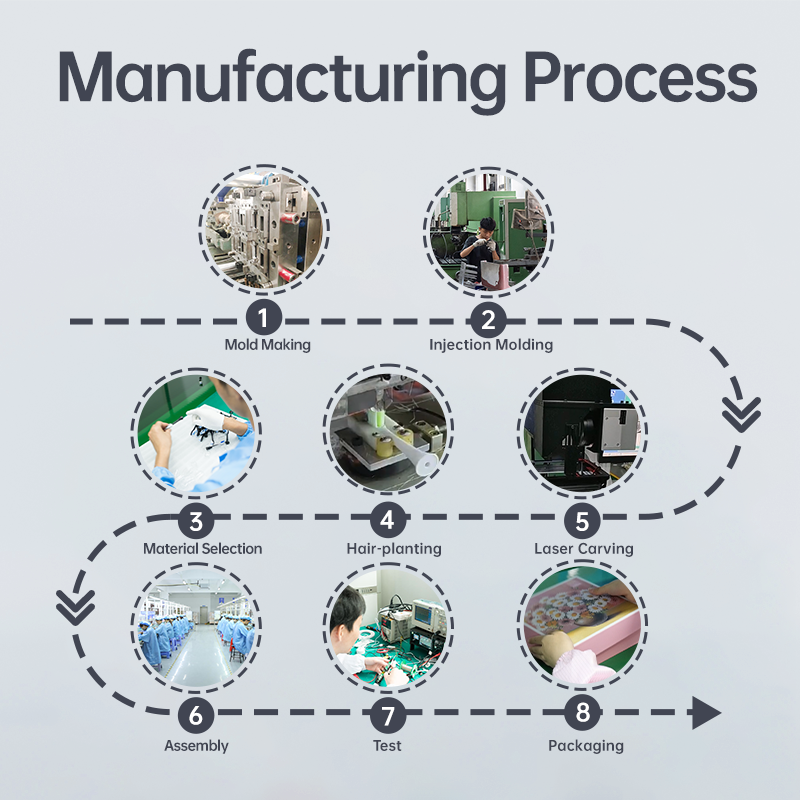
Does POWSMART Multi-Function Toothbrush Cause User Adaptation Struggles?

Maintenance tips for electric toothbrushes

Why Is Bristle Configuration Design Critical When Developing a Brush Handle Mold for Ergonomic Toothbrushes?

How Changing Consumer Attitudes Are Shaping Oral Care Product Demand

The Rise of Wireless Teeth Whitening Devices: Manufacturing Innovations

Waterproof Electric Toothbrush OEM Solutions for Healthcare Workers in Texas
.jpg)
Is Your Oral Care Gentle Enough?

How Dentists Start an Electric Toothbrush Business Introduction
.jpg)
Did you know a magnetic-levitation sonic motor can deliver 31,000 powerful cleaning strokes per minute?

How Much Do You Know About the Knowledge of the Water Pump?