For oral care devices and professional hygiene equipment, Pressure Stabilization is a critical function that directly affects safety, performance, and user confidence. However, even the most advanced technology can underperform if end users do not understand how to operate it correctly. This is why a clearly written and well-structured User Manual plays a vital role in ensuring proper use, especially in B2B-distributed products that serve diverse markets and user profiles.
Clear instructions in the User Manual guide users on how Pressure Stabilization works and when it is activated. Without proper explanation, users may apply incorrect pressure settings, leading to inconsistent results or potential device misuse.
Pressure Stabilization systems designed to adapt to different operating conditions. A detailed User Manual helps standardize user behavior, ensuring that the feature performs consistently whether the device is used in clinical, professional, or home environments.
When Pressure Stabilization limits clearly explained, users gain confidence in the device’s safety mechanisms. Transparent documentation reassures buyers and end users that the product engineered to protect both the device and the user during operation.
For B2B manufacturers, unclear documentation often results in higher customer service demand. A comprehensive User Manual reduces technical inquiries by clearly outlining setup, calibration, and pressure-related precautions, lowering long-term support costs.
In many markets, proper documentation is a regulatory requirement. Clearly defining Pressure Stabilization behavior within the User Manual helps demonstrate compliance with safety standards and supports smoother certification and audit processes.
A well-designed User Manual reflects the manufacturer’s commitment to quality. Clear explanations of advanced features like Pressure Stabilization enhance brand credibility and add value for OEM partners seeking reliable, professional-grade solutions.
Yes—clear instruction in the User Manual is vital for proper Pressure Stabilization use. For B2B manufacturers, effective documentation bridges the gap between engineering innovation and real-world application, ensuring safety, compliance, and long-term customer satisfaction while reinforcing brand trust in competitive markets. Contact us

.jpg)
Whitening Device Packaging Customization for OEM and ODM Brands

Houston sonic toothbrush manufacturer
.jpg)
Smart Timer Electric Toothbrush Bulk | High-Accuracy Brushing Timer for OEM Orders

Can Smart App Connectivity Be Leveraged to Manage Brush Head Replacement Supply?
.jpg)
Portable Water Flosser OEM Solutions for Global Oral Care Brands
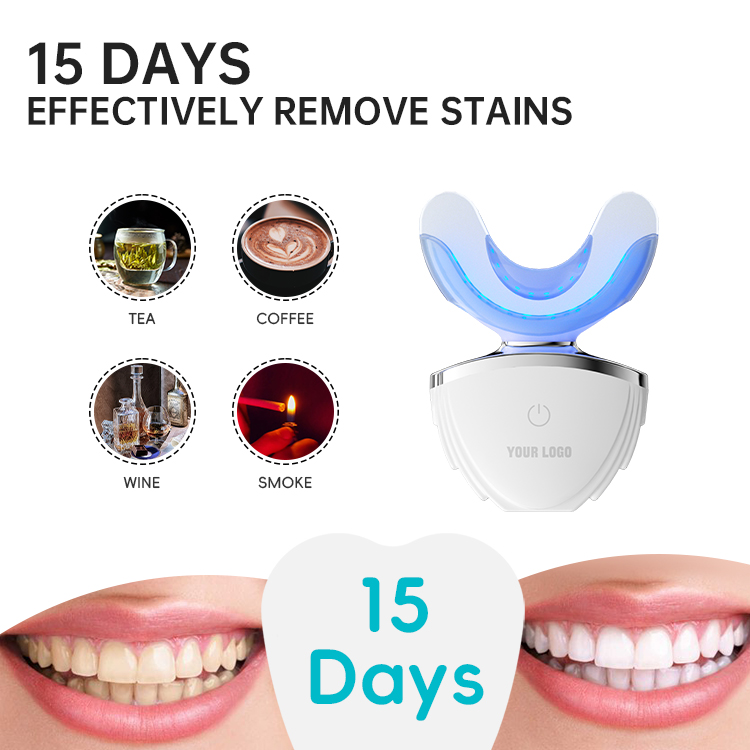
How Is Whitening Device Safety Engineered Into a Portable Whitening Kit?
.jpg)
Orthodontic Electric Toothbrush OEM: Specialized Care Solutions
.jpg)
sonic electric toothbrush Houston
.jpg)
Why Partner with a Water Flosser Supplier for a Multi-Function Flosser with Massage Modes?

How Does Advanced Whitening Gel Formulation Cater to Sensitive Teeth Whitening Needs?
.jpg)
Customized Sonic Toothbrush OEM Supplier for Global Brands
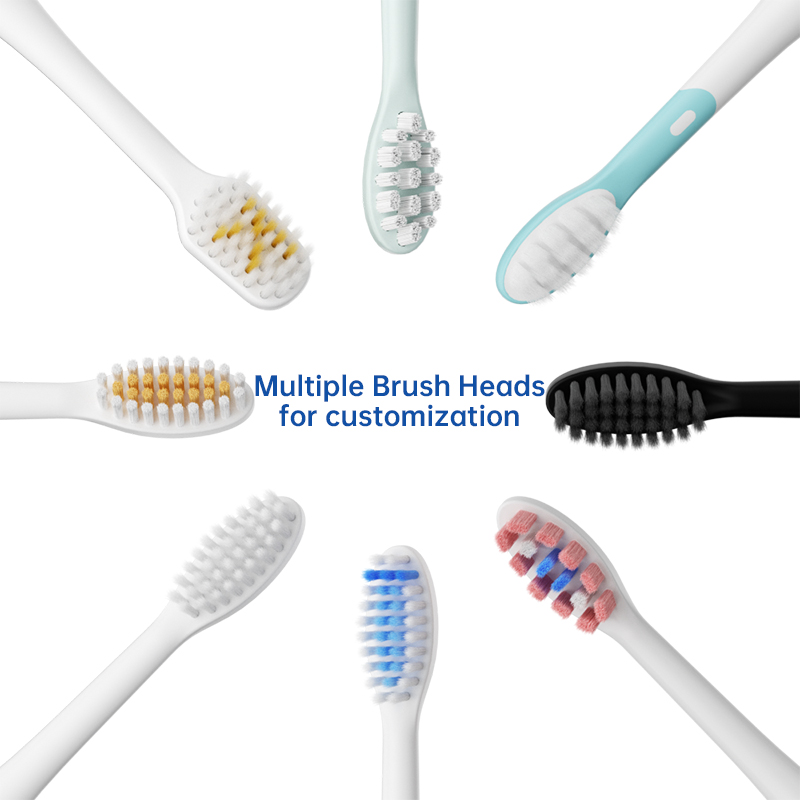
Extend Electric Toothbrush Head Life: An OEM Strategy for Customer Satisfaction

Deliver 7-Day Whitening: Partner with an OEM for Proven Oral Care Products

Multi-Packs and Family Sets – Electric Toothbrush OEM Packaging
.jpg)
Electric Toothbrush for Braces Patients OEM | Orthodontic-Friendly Sonic Brush Supplier

Can an Electric Toothbrush Designer Drive Smart Toothbrush Innovation with AI Features?