In professional oral care devices, pH imbalance and biofilm regrowth are two persistent yet often underestimated challenges. Manufacturers typically address these issues separately. However, mounting evidence suggests that these two phenomena may form a vicious cycle — where improper pH control fosters biofilm regrowth, which in turn further destabilizes pH levels. Could this cyclical problem be undermining device hygiene and patient outcomes? This article explores the mechanisms and solutions.
pH imbalance refers to deviations from the optimal pH range (typically between 5.5 and 7.0) within device fluid systems. In oral irrigators, whitening applicators, or water flossers, pH instability can result from:
When fluids remain too acidic or alkaline, they not only irritate tissues but also disrupt normal biofilm control mechanisms.
Biofilm regrowth occurs when bacterial communities re-establish themselves on internal surfaces of oral care devices after incomplete cleaning. Factors promoting regrowth include:
Biofilms not only compromise device hygiene but also become reservoirs for pathogens, posing infection risks. Company web:https://www.powsmart.com/product/electric-toothbrush/
The connection between pH imbalance and biofilm regrowth is often overlooked:
Thus, pH shifts and biofilm expansion perpetuate each other, locking devices into a self-sustaining contamination loop.
Several design and production oversights exacerbate this vicious cycle:
These factors collectively promote both chemical instability and microbial regrowth.
Manufacturers aiming to break the cycle must address both root causes simultaneously:
Together, these strategies prevent conditions that allow either biofilm regrowth or pH imbalance to dominate.
For B2B customers, eliminating this vicious cycle delivers tangible advantages:
Solving this hidden systemic flaw enhances both functional reliability and market reputation.
Is there a vicious cycle between pH imbalance and biofilm regrowth? Absolutely. For oral care device manufacturers, recognizing and breaking this cycle through material selection, structural optimization, and integrated chemical management is essential to ensuring product safety and hygiene. Contact us
.jpg)
.jpg)

How to Control the Life of Key Components of Water Flosser and Reduce the After-Sales Rate?
.jpg)
Custom Electric Toothbrush Factory for OEM and Private Label Brands
.jpg)
Portable Mini Water Flosser Supplier

Is the Water Flosser Worth Buying?

Cheap vs High-End Electric Toothbrushes : 5 Key Differences You Should Know

Does the Nozzle of the Water Flosser Need to Be Changed Regularly?

Can a soft-bristle brush head designed for sensitive gums make your brushing process more comfortable?
.jpg)
Need funding? Dental grants for WA clinics – how to apply
.jpg)
Micro-Vibration Toothbrush OEM | Precision Sonic Cleaning for Global Distributors
.jpg)
Austin Electric Toothbrush Wholesale | Retail Growth Guide
.jpg)
Thanksgiving Sonic Toothbrush Client Gift | Wellness Appreciation
.jpg)
Electric Toothbrush Gift Set Supplier | Premium B2B Oral Care Gifts

Seeking Electric Toothbrush Innovation Partners Who Are Electric Toothbrush Sustainable Supplier?
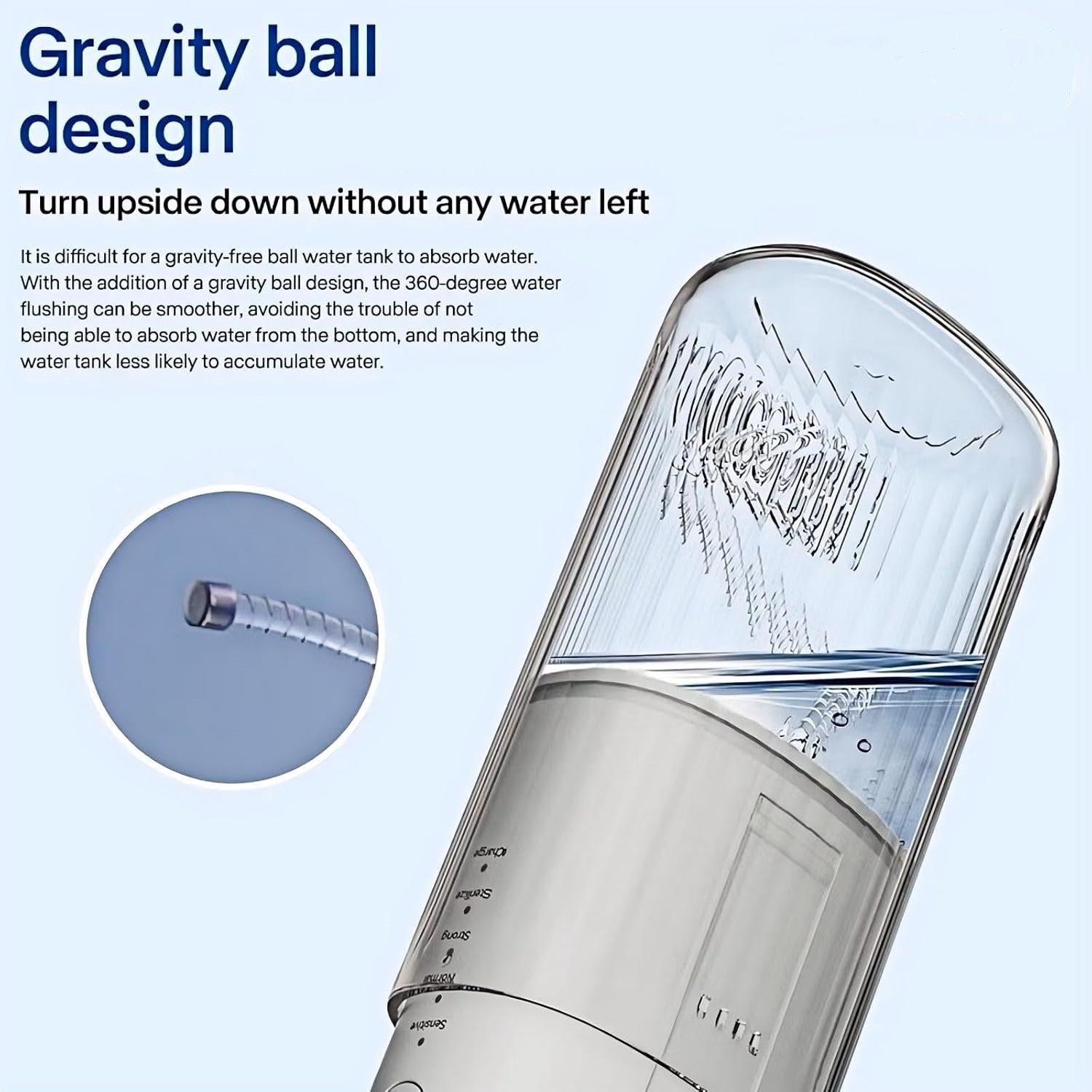
Causes and Solutions to Water Leakage Problems of Water Flossers

Building Quality Water Flossers: Essential Standards for Home Use & OEM Production
.jpg)
Can UV Light Degradation Affect Whitening Gel Leakage?