In the global beauty and medical-aesthetic market, regulatory credibility is no longer optional. An FDA Approved Device plays a central role in achieving full Aesthetic Device Compliance, especially for brands seeking long-term growth in regulated markets such as the United States. FDA alignment not only validates product safety and effectiveness but also strengthens market access and brand trust.
For many distributors, clinics, and retailers, an FDA Approved Device is a baseline requirement. Meeting FDA standards ensures Aesthetic Device Compliance with one of the world’s most recognized regulatory authorities, enabling smoother entry into the US and other compliance-driven regions.
FDA approval requires documented testing, risk analysis, and quality controls. An FDA Approved Device demonstrates that the product meets strict safety and performance benchmarks, reinforcing Aesthetic Device Compliance and reducing concerns related to user injury, misuse, or adverse effects.
Non-compliant devices expose brands to recalls, import holds, and legal penalties. By prioritizing FDA Approved Device pathways, manufacturers significantly lower regulatory risk while ensuring ongoing Aesthetic Device Compliance throughout the product lifecycle.
Clinics, dermatology practices, and aesthetic professionals prefer devices with verified regulatory status. An FDA Approved increases confidence among professional users and simplifies procurement decisions, reinforcing Aesthetic Device Compliance in clinical and semi-clinical environments.
From a manufacturing perspective, FDA-aligned processes support consistent documentation, traceability, and quality management. This makes it easier for OEM/ODM partners to customize an FDA Approved.
In a crowded aesthetic device market, regulatory transparency is a powerful differentiator. Promoting an FDA signals commitment to quality, safety, and compliance, helping brands position themselves as reliable leaders in Aesthetic Device Compliance.
Achieving Aesthetic Device Compliance starts with regulatory credibility. By developing and marketing an FDA Approved, brands and manufacturers can ensure safer products, smoother market access. Contact us


Electric Toothbrush & Water Flosser Combination Set: How to Increase the Average Transaction Value through Scenario-Based oral care products Design?
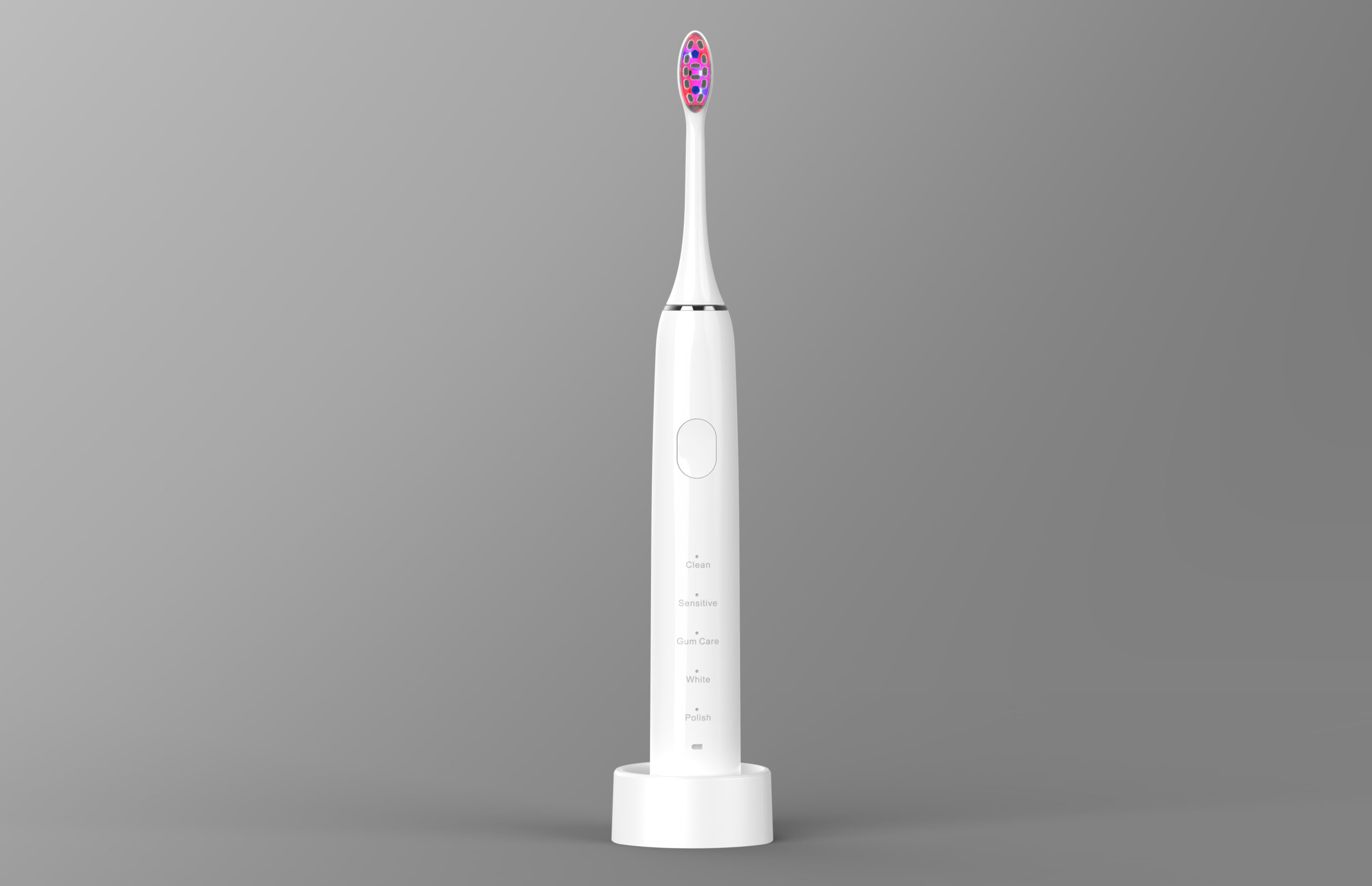
Can a Portable Water Flosser incorporate elements of Desktop Irrigator Design for versatility?

Why Smart Electric Toothbrushes Are Oral Care Market Trends and in Demand
.jpg)
Looking for Electric Toothbrush for Travel Wholesale as an Oral-B Electric Toothbrush Distributor?

Marketing Support from OEM Partners: Beyond Manufacturing
.jpg)
Whitening Gel Compatibility Device Considerations for OEM Whitening Brands
.jpg)
OEM Water Flosser Manufacturing: Custom Solutions for Brands

The Competitive Edge: How Water Flosser brands Stay Ahead in the Market
Which Electric Toothbrush Is Best for College Students in Boston?

How Long Should I Use Blue Light on My Teeth? OEM Manufacturer’s Guide

Why Do Some Electric Toothbrush Make a Lot of Noise?

Can I Use an Electric Toothbrush in the Shower?
.jpg)
Why Choose an Orthodontic Water Flosser Featuring Multi-pressure Setting Flosser Capabilities?
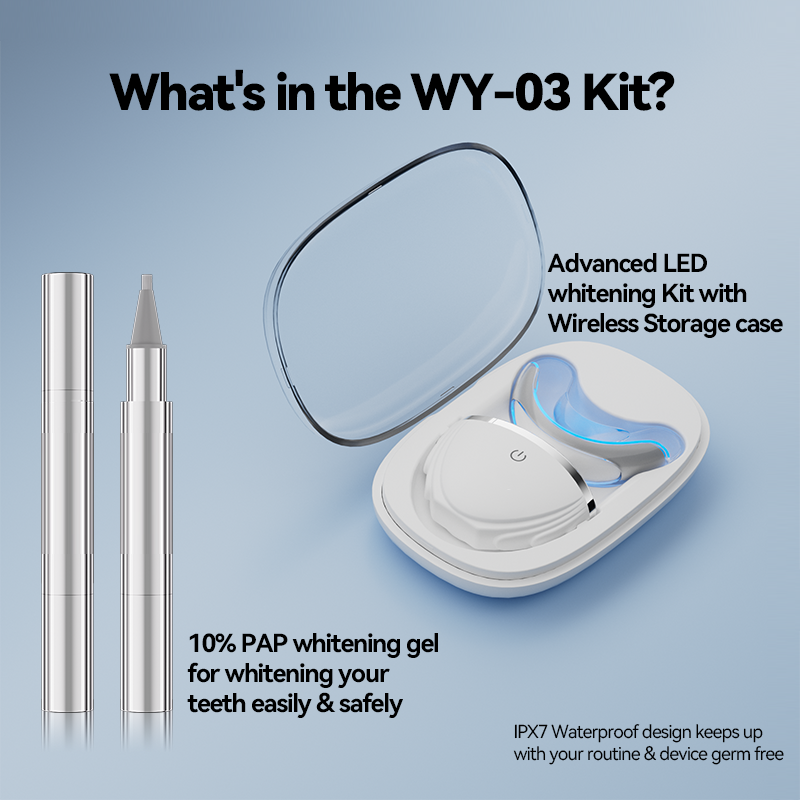
Can a Whitening Pen Supplier Deliver an Effective At-Home Whitening Solution for Busy Consumers?
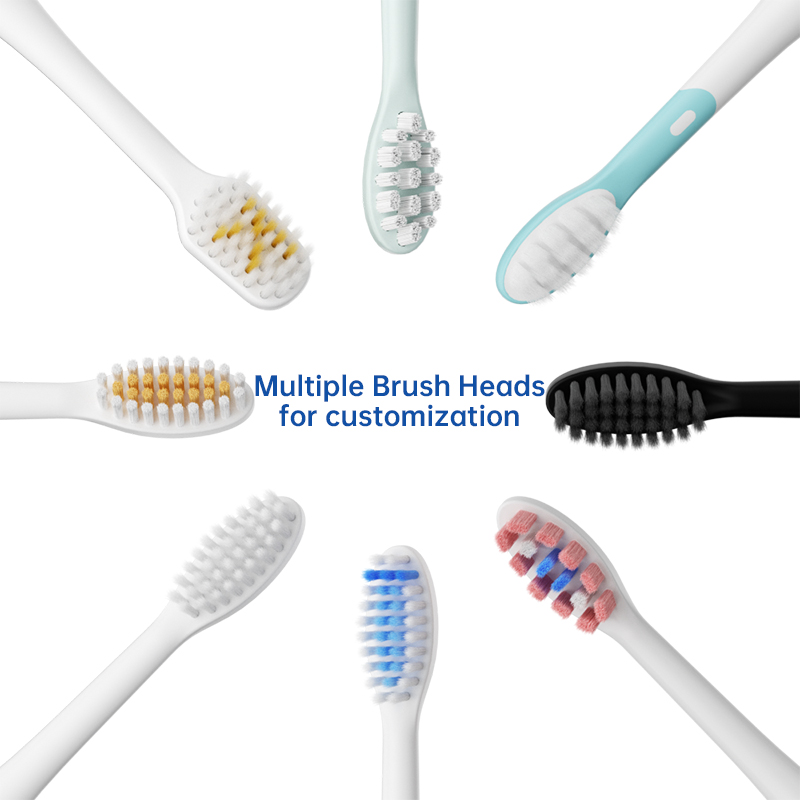
Looking for a China electric toothbrush that’s also a smart sonic toothbrush?

Can Smart App Connectivity Enhance the User Feedback from Pressure Sensor Calibration Data?