In the highly regulated beauty and personal care sector, Packaging Customization Service plays a critical role in supporting Aesthetic Device Compliance. Beyond visual branding, packaging must align with regulatory labeling, safety communication, and regional market requirements. For B2B manufacturers and OEM/ODM partners, customized packaging becomes an essential compliance enabler rather than a purely aesthetic choice.
Aesthetic devices often require specific handling and storage conditions. Through a professional Packaging Customization Service, packaging structures can be designed to protect sensitive components, prevent misuse, and support safe transportation—directly contributing to Aesthetic Device Compliance.
Different markets impose strict rules on labeling, symbols, and multilingual instructions. Customized packaging layouts ensure that compliance-critical information is clearly displayed, properly sized, and correctly positioned, helping brands meet Aesthetic Device Compliance standards across regions.
Packaging is often the first point of user interaction. A tailored Packaging Customization Service allows manufacturers to integrate warnings, usage diagrams, and precaution statements directly into the packaging design, reducing misuse risk and reinforcing compliance expectations for aesthetic devices.
Compliance requirements vary significantly between markets such as the EU, US, and Asia. With a flexible Packaging Customization Service, OEM/ODM partners can localize packaging elements—materials, markings, and languages—while maintaining consistent Aesthetic Device Compliance across product lines.
Customized packaging can incorporate batch numbers, QR codes, or traceability labels. These features support post-market surveillance and recall readiness, which are increasingly important aspects of Aesthetic Device Compliance for professional and consumer-use devices.
When packaging designed in parallel with the device itself, compliance documentation and testing can proceed more efficiently. A well-integrated Packaging Customization Service reduces rework, minimizes approval delays, and helps brands achieve faster, smoother compliance validation.
A well-executed Packaging Customization Service is a strategic asset in achieving and maintaining Aesthetic Device Compliance. For B2B manufacturers and OEM/ODM partners, customized packaging not only enhances brand presentation. Contact us

.jpg)
Can a Houston graduation care package include a premium toothbrush?
.jpg)
Is a Reliable Water Flosser Pump Supplier Key to Developing a High-Pressure Flosser?
.jpg)
Oral Care Products Supplier in Houston TX

Does Jet Stream Precision Depend on Long-Term Hose Durability?

Seeking Custom Handle Design Services Within Your OEM/ODM Manufacturing Partnership?
.jpg)
OEM Electric Toothbrush Supplier Supporting Small Orders

Is Clear Instruction in the User Manual Vital for Proper Pressure Stabilization Use?
.jpg)
How Does a Water Flosser OEM Implement Quiet Flosser Technology for Home Use?
.jpg)
USB-C Rechargeable Oral Irrigator Manufacturer | OEM for Portable Water Flossers
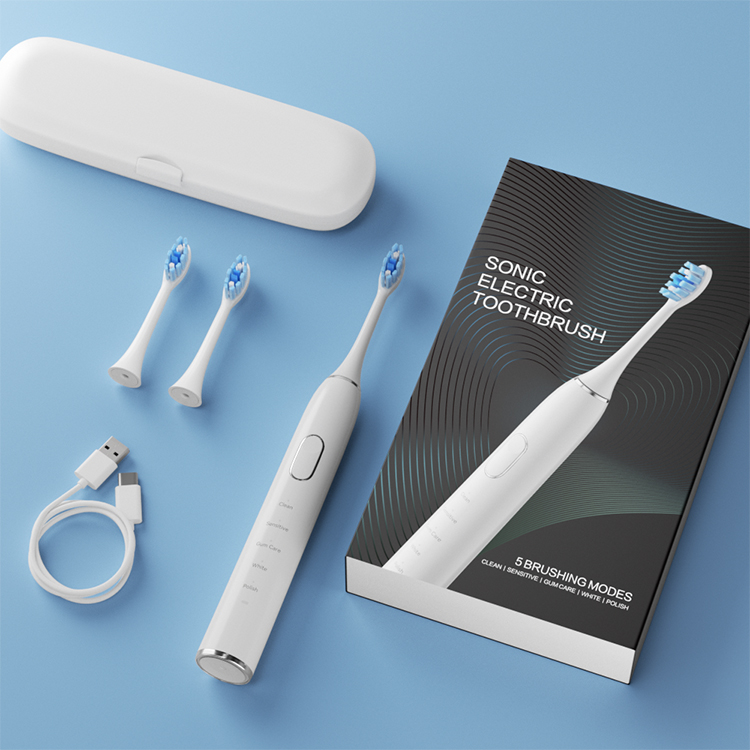
Why Is Cavitation Cleaning Technology More Effective With a Micro-Perforated Brush Head?
.jpg)
Long Battery Life Electric Toothbrush OEM Supplier

ADA Accepted Electric Toothbrush OEM – Certification Support
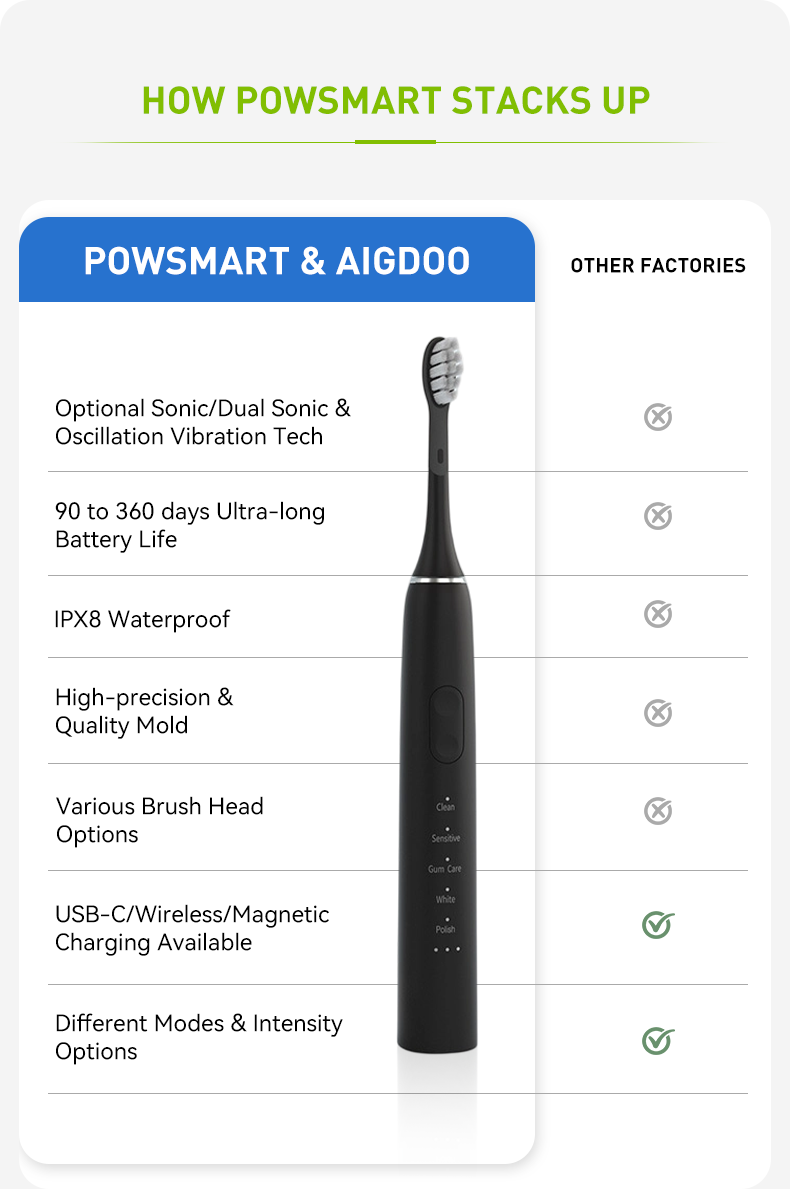
Why Is Universal Brush Head Compatibility a Challenge for Certification Compliance?

How Can I Charge My UK Electric Toothbrush in the USA? Universal Charging Solutions from an OEM Factory
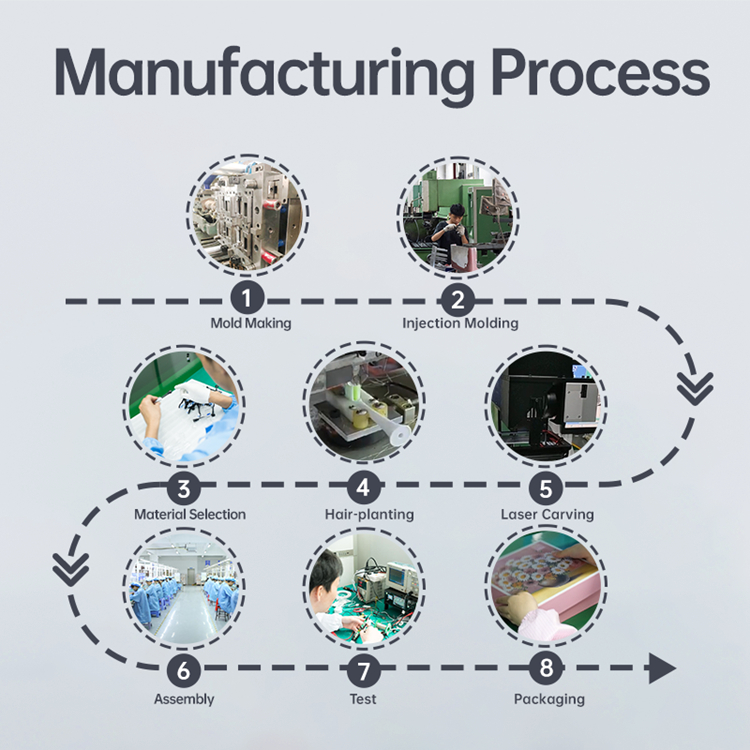
Sourcing Wireless Charging Coils? Need a Reliable Global Logistics Partner for Delivery?
.jpg)
Buy sonic electric toothbrushes in bulk Houston