If you manufacture electric toothbrushes and want clinics, retailers, or procurement teams in the U.S. to accept your products as ADA-ready or ADA-referenced items, partnering with the right US toothbrush distributor can make the difference between a slow, costly launch and a predictable, compliant rollout. Below are six clear reasons — with practical next steps — explaining why a U.S. distributor matters for anything tied to “ADA toothbrush” positioning and electric-toothbrush B2B success.
Making an “ADA toothbrush” claim (or seeking ADA-aligned marketing) involves more than a catchy label — it requires documentation, appropriate testing, and careful claim language that fits U.S. expectations. A US toothbrush distributor understands U.S. labeling norms, advertising rules, and the types of lab/clinical evidence U.S. dental professionals respect.
Therefore: work with a distributor who can review your claims copy, advise on what proof is needed, and recommend accredited U.S. labs or clinical partners for validation.
In addition, clinics and dental networks want to try demo units quickly. A domestic distributor warehouses demo stock, ships same-day samples to key opinion leaders, and can arrange local pilot programs far faster than overseas suppliers. That speed turns pilot enthusiasm into practice adoption — a vital step for electric toothbrushes marketed for professional endorsement.
Consequently: choose a distributor with demo-unit inventory and a track record of clinic pilot coordination.
Moreover, U.S. distributors often act as the coordinating node between manufacturers and U.S. test labs (electrical safety, EMC, and any microbiology or efficacy testing linked to ADA-style claims). They can collect and translate foreign test reports, order additional U.S. testing if needed, and assemble a compliance pack that procurement teams accept.
Action: ask prospective distributors if they provide a “compliance packet” service (translations, lab liaison, and certificate collation).
When you sell electric toothbrushes to clinics or retail, after-sales service matters: warranty claims, battery issues, and spare-head logistics all impact reputation. A U.S. distributor handles RMAs, refurb/replacement flows, and local warranty fulfillment — which reduces friction for dental offices that expect quick turnarounds.
Thus: prefer distributors with U.S. returns hubs and clear SLA metrics for RMA turnaround.
Beyond compliance, a distributor with existing relationships in the dental channel (group purchasing orgs, dental chains, regional dental reps) helps position your product as an “ADA toothbrush” candidate in the right forums. They can package co-branded education kits, arrange continuing-education demos, and supply patient-education collateral that supports clinical recommendations.
In short: a distributor that knows dental marketing shortens the trust curve between your product and clinicians.
Finally, the mechanics matter: customs entry, battery shipping rules, and state tax/regulatory variations complicate cross-border sales. A U.S. toothbrush distributor already manages import compliance, proper labeling for U.S. distribution, and fast replenishment so clinics don’t run out of demo or retail stock. This reliability supports long-term ADA-focused adoption because practices won’t risk recommending a product they can’t re-order quickly.
Therefore: verify the distributor’s import experience (battery handling, TARIC/HTS codes, and bonded warehousing).
Working through a reputable US toothbrush distributor turns “ADA toothbrush” aspirations from marketing copy into a workable B2B strategy: faster pilots, credible documentation, local after-sales support, and stronger clinical adoption. If you’d like, I can draft a one-page distributor RFP that includes the checklist above and tailored questions for electric-toothbrush manufacturers — ready to send to potential partners. Which distribution region in the U.S. should the RFP target? Contact Powsmart
.jpg)
.jpg)
.jpg)
How RV long-life battery defines RV power toothbrush reliability
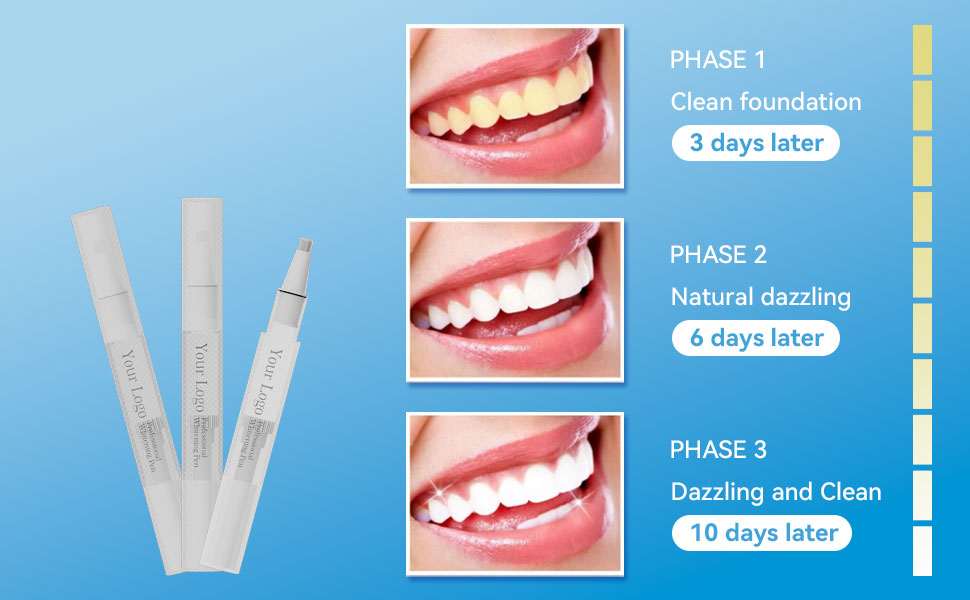
What is LED Teeth Whitening Kit and Usage Guide
.jpg)
Why Choose ADA Approved Electric Toothbrush in Chicago?
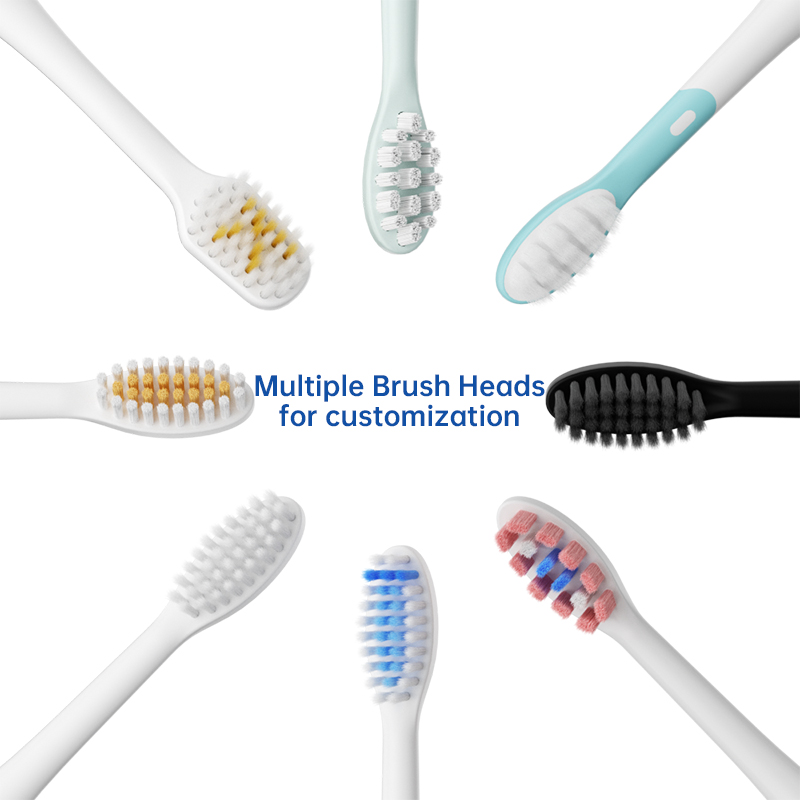
POWSMART USA – Innovative Sonic & Smart Electric Toothbrush Manufacturer
.jpg)
Where to test a Seattle urban toothbrush? Visit the Seattle demo store!
.jpg)
Why Boston clinic recommended brushes help Boston sensitive gums

The Pros and Cons of Wireless Charging in Electric Toothbrushes

sonic electric toothbrush USA
.jpg)
Ergonomic Design in Electric Toothbrushes: OEM Best Practices
Occlusal Interference Plus Jaw Fatigue – Design Flaw?
Nozzle Clogs + Water Leakage — Is Your Irrigator Still Usable?
Occlusal Discomfort Plus Salivary Alteration – Alarming?

Water Flosser vs. Traditional Floss: Pros, Cons & Usage Tips
.jpg)
Hawaii waterproof toothbrush vs. Hawaii beach toothbrush — which model makes sense for snorkeling trips?

The Advantages and Current Usage of Electric Toothbrushes’ cleaning efficincy
.jpg)
Best Electric Toothbrush With Pressure Sensor in Houston?

electric toothbrush heads Ultra Soft

electric toothbrush heads Deep Clean

electric toothbrush heads Regular Clean

Private Label Whitening Gel

Electric toothbrush heads Charcoal Infused-Diamond

electric toothbrush heads Charcoal Infuse-Round
.jpg)
Florida Electric Toothbrush – Powsmart PTR-C8

Customization Teeth Whitening Gel
whstapp
whstapp
National Toll-Free Service Hotline
+86 755 86238638