In the competitive oral beauty and personal care market, whitening device quality control is not just about preventing defects—it’s about protecting your brand, building trust with end users, and meeting global compliance standards. For manufacturers of blue light teeth whitening devices, ensuring a low-defect rate requires a systematic and comprehensive approach to quality management. In this article, we’ll explore five critical steps every factory should follow, including manufacturer quality inspection processes, FDA certification, and rigorous reliability testing protocols.
Quality starts with the materials. From medical-grade silicone to LED chips and batteries, every component must be sourced from verified suppliers. A high-quality whitening device requires:
Strict incoming quality control (IQC) helps prevent hidden issues before assembly begins.
During production, consistency is key. A reliable whitening device quality control process includes:
These processes ensure the physical structure, internal wiring, and charging functions of each device meet precise tolerances—reducing both cosmetic and functional defects.
A two-level inspection system is essential to catch problems early and late in the process:
Professional manufacturers implement AQL (Acceptable Quality Limit) sampling based on international inspection standards.
Even a perfect device in the factory may fail under real-world conditions. That’s why reliability testing is critical to long-term performance and customer satisfaction. Key reliability tests include:
These tests reduce warranty claims and boost brand reputation by ensuring real-world durability.
To sell teeth whitening devices internationally, regulatory compliance is non-negotiable. Obtaining FDA certification, CE marking, or other regulatory approvals assures your partners and end users that the product is safe and effective.
Quality control systems should support:
For professional manufacturers of blue light teeth whitening devices, minimizing defect rates is not just about catching errors—it’s about building a culture of quality from sourcing to shipment. Through:
Verified component sourcing
Controlled production processes
Thorough inspection procedures
Rigorous reliability testing
International FDA certification and compliance
You can achieve a consistently low defect rate and deliver high-performance, trustworthy products to global markets.
Looking for a manufacturer with strong quality control systems? Contact us to learn how our factory ensures top-tier product reliability for your brand through OEM/ODM services.

As Young People Pay More Attention to Their Teeth, What New Trends Are Emerging in Oral Care?
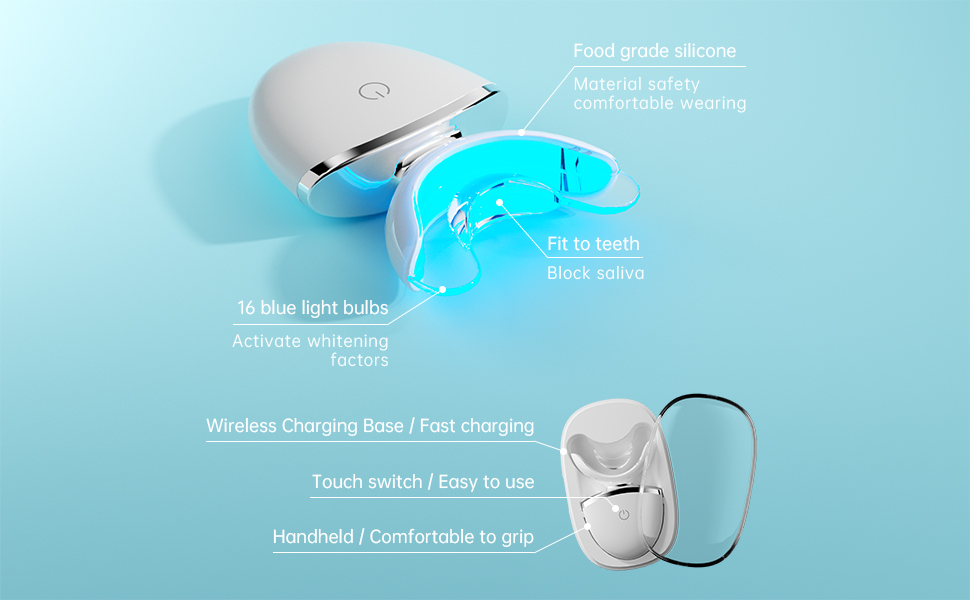
How Long Should You Leave Blue Light on Your Teeth? OEM Manufacturer’s Guide

Is Blue Light Under 480nm Safe for Teeth Whitening Devices?

Professional vs. Home Use: Key Technical Differences in Teeth Whitening Blue Lights

How Often Should I Use a LED Teeth Whitening Light? A Manufacturer’s Guide
.jpg)
Sync Disruption with Tray Deformation – Tech Failure?
.jpg)
Sync Errors Causing Taste Distortion – Tech Glitch?

Private mold development of Red and Blue Light Teeth Whitening Device: Shell Material Selection and Ergonomic Design Points
.jpg)
Portable LED Teeth Whitening Device Supplier
.jpg)
Explaining the Teeth Whitening Device and How to Use It?
.jpg)
Factory Supplier for Waterproof Sonic Toothbrushes

Uncovering Dentistry: Scientific Methods and Techniques for Whiter Teeth
.jpg)
Portable LED Teeth Whitening Kit OEM

Case Study of Intelligent Upgrading of Dental Instrument Factory
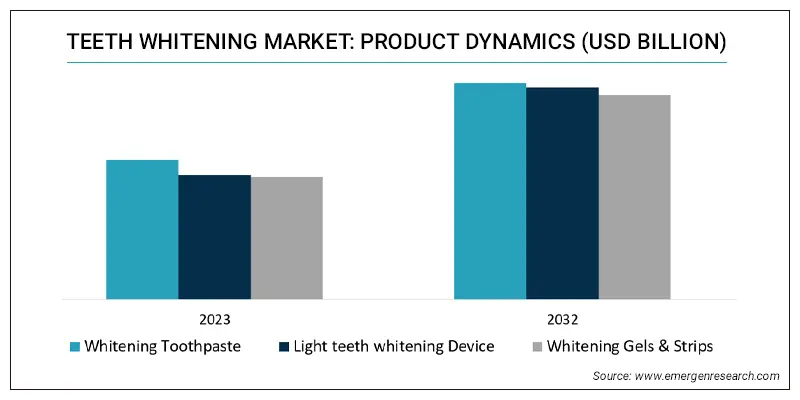
Analysis of Teeth Whitening Device Market Trends: The Functions and Designs That Consumers Will Pay Most Attention to in 2025
.jpg)
Does Teeth Sensitivity Worsen Tetracycline Stains? New Whitening Side Effects!