Material degradation in oral care devices, such as electric toothbrushes and water flossers, is an often-overlooked issue. Yet, it can quietly lead to the release of harmful chemical residue into the user’s mouth, raising significant health concerns. For B2B manufacturers, this is not just a matter of consumer trust but also a critical compliance and product lifecycle issue. This blog will dissect the causes, risks, and prevention strategies related to material breakdown and chemical contamination in oral hygiene devices.
Material degradation refers to the physical or chemical breakdown of components over time due to factors like:
As the material degrades, microscopic particles or chemical residue may leach from brush heads, water tanks, seals, or internal tubing—especially in low-cost devices or poorly stored stock.
When chemical residue enters the mouth, it may:
For children and users with oral ulcers or compromised immunity, the consequences may be more severe, requiring clinical attention. Company web: https://www.powsmart.com/product/electric-toothbrush/
The following design and manufacturing oversights can significantly speed up material degradation:
In addition, devices stored long-term in high-humidity environments, such as warehouse stock without climate control, are highly vulnerable.
Manufacturers and distributors should monitor for these early indicators:
These signs often precede more severe health-related reports, making proactive detection vital.
To reduce the risk of chemical residue caused by material degradation, consider the following:
These enhancements not only protect end users but also reduce warranty claims and ensure smoother regulatory approvals.
Regulatory bodies such as the FDA, CE, and China’s NMPA are paying increasing attention to chemical safety in consumer products. Failing to manage material degradation risks may lead to:
Thus, investing in safe materials isn’t just ethical—it’s a strategic safeguard.
Material degradation and the resulting chemical residue are silent threats that can compromise both product safety and business credibility. For B2B oral care device manufacturers, early attention to material selection, chemical compatibility, and quality control processes is essential. At our manufacturing facility, we integrate advanced testing and certified materials to ensure every product delivered is safe, durable, and regulation-ready. Contact us
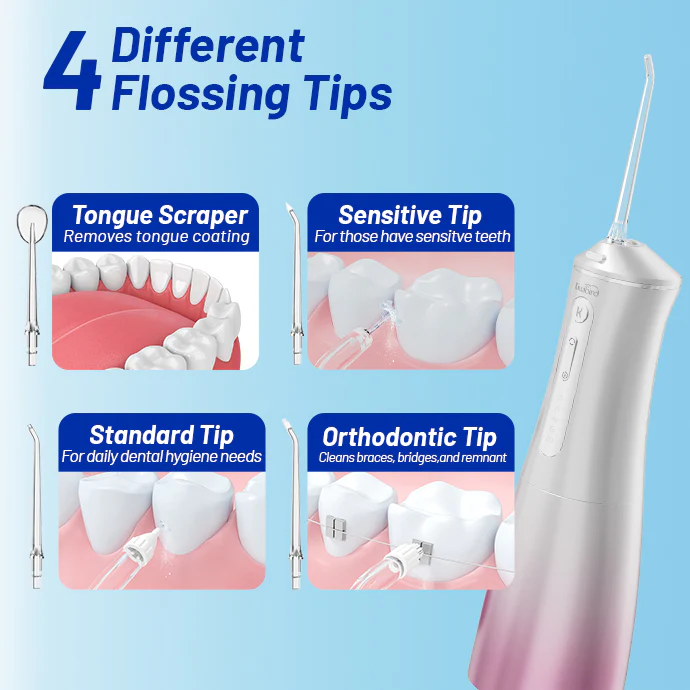

Antibacterial Water Flosser Technology Trend: The Importance of UV Sterilization for Water Tank Hygiene Assurance
Why Does Waterproof Failure Cause Pressure Fluctuation in Water Flossers?
.jpg)
Travel-Friendly Water Flosser: From Foldable Nozzles to Globally Voltage-Compatible Solutions
Does a Water Flosser Require Regular Maintenance? How Should It Be Cleaned?

Gingival Abrasion with Saliva Depletion – A Silent Crisis in Oral Care Design?

How to Achieve a Win-Win Situation of “Consumption Upgrade” and “Cost Control”?
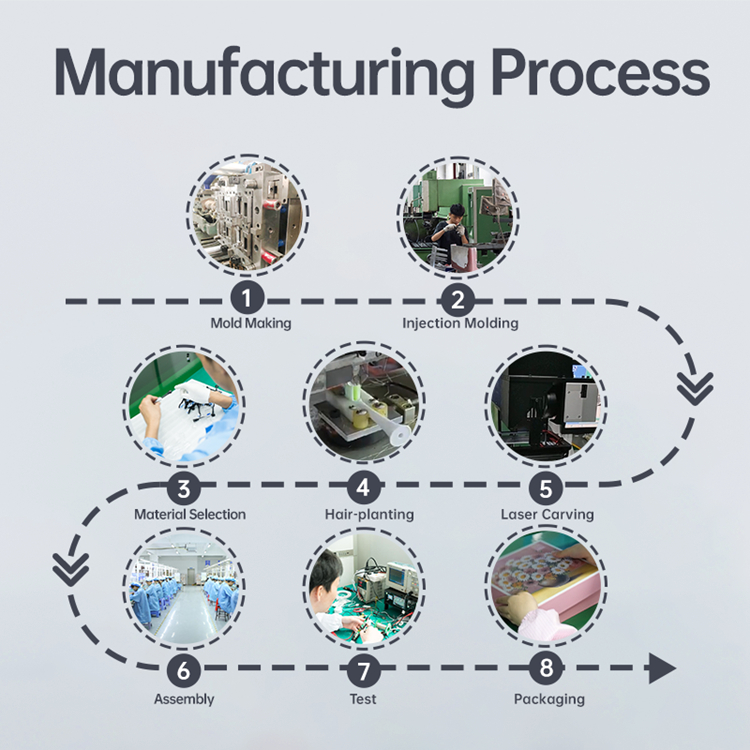
Choosing Electric Toothbrush Handle Processes: An OEM Guide to Cost vs. Quality

The Market Potential of Teeth Whitening: The Consumer Psychology Behind Annual Growth of Home Teeth Whitening Devices

Can a Folding Toothbrush Design Make This Travel Electric Toothbrush Fit in a Wallet?

Oral Care and Personal Confidence Enhancement: A Dual Strategy from Whitening Effect to Product Appearance Level
.jpg)
Need a Transparent Electric Toothbrush Cost Breakdown to Build a Strong Electric Toothbrush Supplier Relationship?

How Can oral care Brand Owners Enhance brand influenceThrough Product Expansion Strategies?
.jpg)
Is Bluetooth Toothbrush APP Tracking Failure Causing User Adaptation Issues?
.jpg)
How to Evaluate an Electric Toothbrush Supplier Portfolio and Electric Toothbrush Supplier Capabilities?
.jpg)
Is Your POWSMART Smart Rechargeable Toothbrush Leaking Battery?

Can Travel Electric Toothbrush’s Waterproof Failure Cause Circuit Corrosion?

Customization Teeth Whitening Gel

Electric toothbrush heads Charcoal Infused-Diamond

electric toothbrush heads Ultra Soft

electric toothbrush heads Charcoal Infuse-Round

electric toothbrush heads Deep Clean
.jpg)
Florida Electric Toothbrush – Powsmart PTR-C8

Private Label Whitening Gel

electric toothbrush heads Regular Clean
whstapp
whstapp
National Toll-Free Service Hotline
+86 755 86238638