In the dental beauty industry, safety and compliance are crucial for both manufacturers and consumers. To ensure product quality, manufacturers must obtain necessary certifications, such as FDA and CE, before entering global markets. This article explores the FDA and CE certification process for dental beauty devices, helping factories navigate the regulatory landscape.
The U.S. Food and Drug Administration (FDA) regulates medical devices, including dental beauty devices, to ensure they meet safety and performance standards. Depending on the risk classification, manufacturers may need to submit a 510(k) premarket notification or a Premarket Approval (PMA) application.
The CE mark indicates compliance with European Union safety, health, and environmental requirements. To obtain CE certification, manufacturers must comply with Medical Device Regulation (MDR 2017/745) and undergo a conformity assessment, often involving a Notified Body.

Devices are classified based on their potential risks:
FDA: Class I (low risk), Class II (moderate risk, requiring 510(k)), Class III (high risk, requiring PMA).
CE: Class I, IIa, IIb, III, with stricter requirements for higher-risk devices.
To ensure the compliance of dental beauty devices, manufacturers must conduct biocompatibility testing, electrical safety tests (IEC 60601-1), and clinical evaluations to demonstrate product safety.
Compliance requires an effective QMS, such as ISO 13485, which outlines procedures for medical device design, production, and post-market surveillance.
Device Classification – Identify the correct class based on FDA guidelines.
Premarket Submission – Submit a 510(k) or PMA, depending on classification.
Testing and Documentation – Provide test reports, clinical data, and manufacturing details.
FDA Review and Clearance – The FDA reviews the submission, inspects facilities, and grants approval.
Classification and Conformity Route Selection – Identify the correct device class.
Technical Documentation Preparation – Submit a comprehensive dossier with risk analysis and testing results.
Notified Body Assessment – If applicable, a Notified Body reviews and audits the device and QMS.
Declaration of Conformity & CE Marking – Once approved, the manufacturer can affix the CE mark.
Regulatory Updates: Both FDA and CE regulations evolve, requiring continuous compliance efforts.
Clinical Data Requirements: Manufacturers must provide robust clinical evidence for higher-risk devices.
Manufacturing Consistency: Ensuring consistent quality and compliance of dental beauty devices throughout production is essential.
Achieving FDA and CE certification for dental beauty devices is a complex but necessary process for manufacturers entering global markets. By understanding classification, testing, QMS, and submission procedures, factories can ensure compliance and market access. Partnering with regulatory experts can further streamline the approval process, ensuring efficiency and success.https://www.powsmart.com/
.jpg)
Are You the Leading Water Flosser Nozzle Supplier for High-Volume Replacement Head Production?
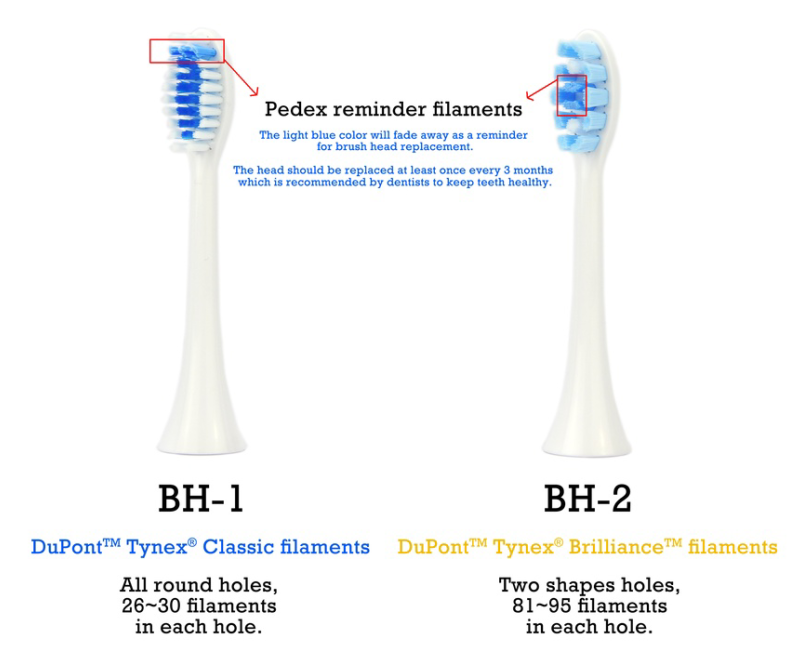
FDA-Approved Antibacterial Bristles of Electric Toothbrush: A Safety Guide for Oral Care
.jpg)
Recyclable Packaging Toothbrush Supplier | Eco-Friendly Oral Care
.jpg)
Florida Electric Toothbrush – Powsmart PTR-C8 for Humid Climates
.jpg)
Timer Malfunction with Whitening Reversal – Wasted Effort?
.jpg)
Rechargeable Oral Irrigator OEM
.jpg)
Electric Toothbrush Cost Breakdown for OEM & Wholesale Buyers

Solutions to Common Problems with Teeth Whitening Devices: How Can Manufacturers Reduce Product Return Rates?

Can Old Yellow Teeth Be Whitened? Effective Solutions from the Manufacturer

How Does a Smart Timer Chip Enhance the User Experience of an Ergonomic Handle Design?
Dental Hygienist’s View on Water Flossers – Key Insights for OEM Product Development

Kids Water Flosser Age Guide: When to Introduce and How to OEM the Right Product

Why choose ABS material for teeth whitening trays
.jpg)
Practical Valentine’s Day Gift for Husband Electric Toothbrush | Powsmart
.jpg)
peroxide free whitening pen wholesale | OEM Non-Peroxide Teeth Whitening Pen Supplier

ADA-Certified Electric Toothbrush Suppliers in Chicago – Compliance & Customization