While the Bacteria Killing Ultrasonic Brush is praised for its sterilization performance and oral hygiene benefits, questions are rising around a less-discussed issue: Reservoir mold. Despite its antimicrobial function at the brush head, improper internal moisture management may trigger unwanted microbial growth in the water reservoir. For OEM/ODM clients and dental care brands, this raises significant design, safety, and user trust concerns.
Ultrasonic brushes with bacteria-killing claims often use UV, blue light, or high-frequency sonic vibration to reduce microbial activity on bristles and contact surfaces. However, water reservoirs—especially in models with integrated flossing or rinsing systems—remain largely stagnant environments if not properly drained or aerated. This creates a breeding ground for reservoir mold, undermining the product’s hygiene promise.
Even in premium Bacteria Killing Ultrasonic Brushes, the following design or usage flaws can lead to mold formation:
These overlooked details can accumulate over weeks, leading to mold patches and unpleasant odors.
Many OEMs still use standard ABS or PC plastics for water chambers. Unless treated with anti-fungal or hydrophobic coatings, these materials easily host biofilm, a precursor to visible reservoir mold. In particular, ultrasonic vibration doesn’t reach the inner walls of sealed reservoirs, making surface material and shape even more important.
To prevent mold-related complaints and improve product longevity, brands should demand the following features from manufacturers:
These small changes dramatically improve real-world performance and reduce after-sale issues.
Even with robust design, reservoir mold can still form if users are not properly informed. OEMs and ODM partners should insist that IFUs (Instructions for Use) include:
User behavior plays a critical role in preventing mold recurrence.
Top-tier factories now implement simulation testing by storing filled reservoirs under controlled humidity and temperature cycles. Mold resistance metrics—such as ASTM G21 fungus testing or ISO 846 microbial growth resistance—should be standard validation tools for new ultrasonic brush models.
The Bacteria Killing Ultrasonic Brush is only as hygienic as its full system design—not just the bristles. Without thoughtful engineering, reservoir mold may become the hidden weakness of an otherwise high-performance product. For B2B buyers and brand managers, prioritizing mold-prevention in structural design, materials, and usage instruction is no longer optional—it’s a competitive necessity. Contact us
-300x300.jpg)
-300x300.jpg)

Top OEM Electric Toothbrush Manufacturer for Kids – Powsmart

Does Wireless Charging Toothbrush IPX7 Release Material Toxicity?

Magnetic Levitation Motor Toothbrush Technology Explained
Is Tooth Sensitivity with Enamel Erosion Whitening’s Side Effect?
-2-scaled.png)
Does 3D Rotation Oscillation Brush Trigger Sensor Failure?
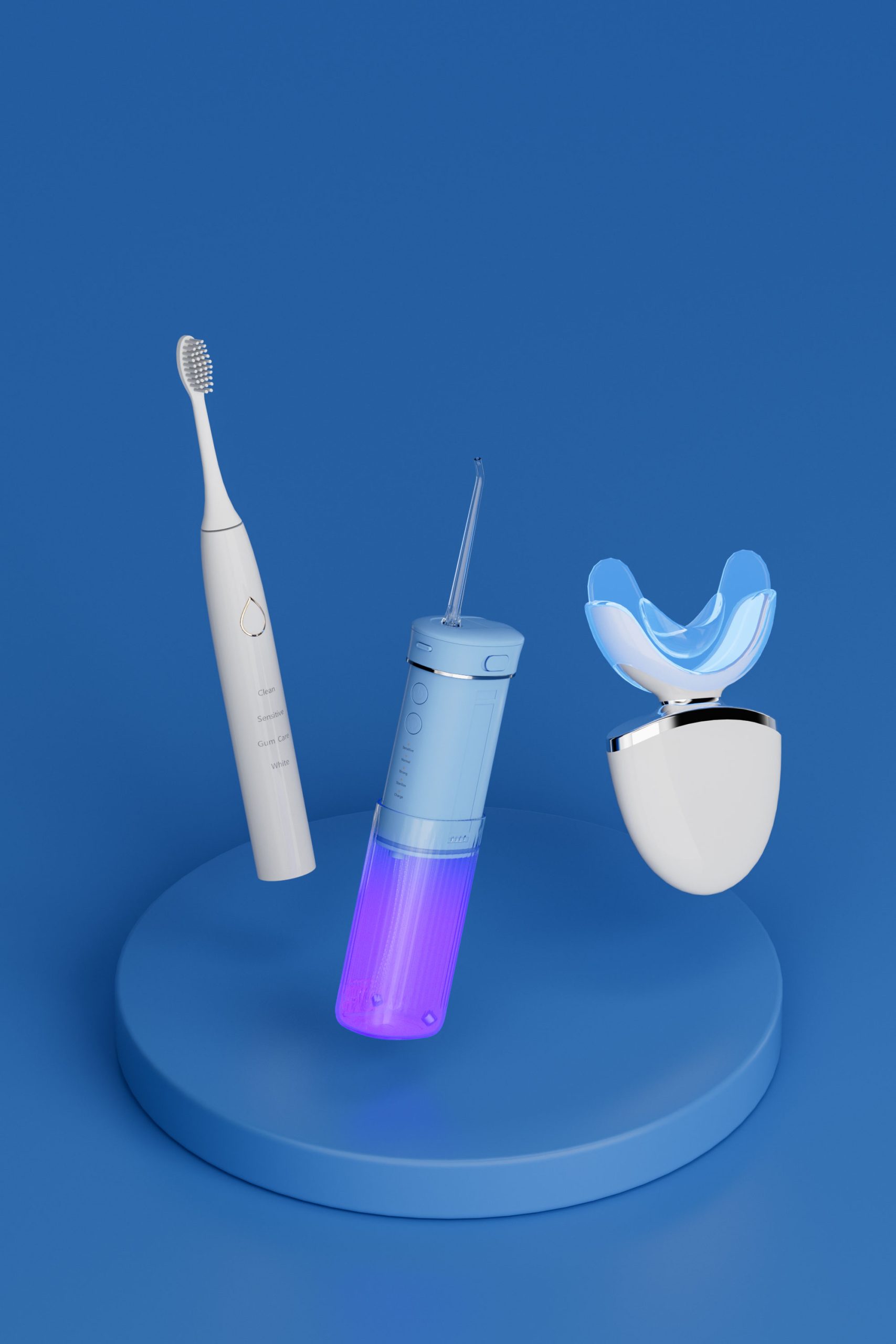
About Powsmart
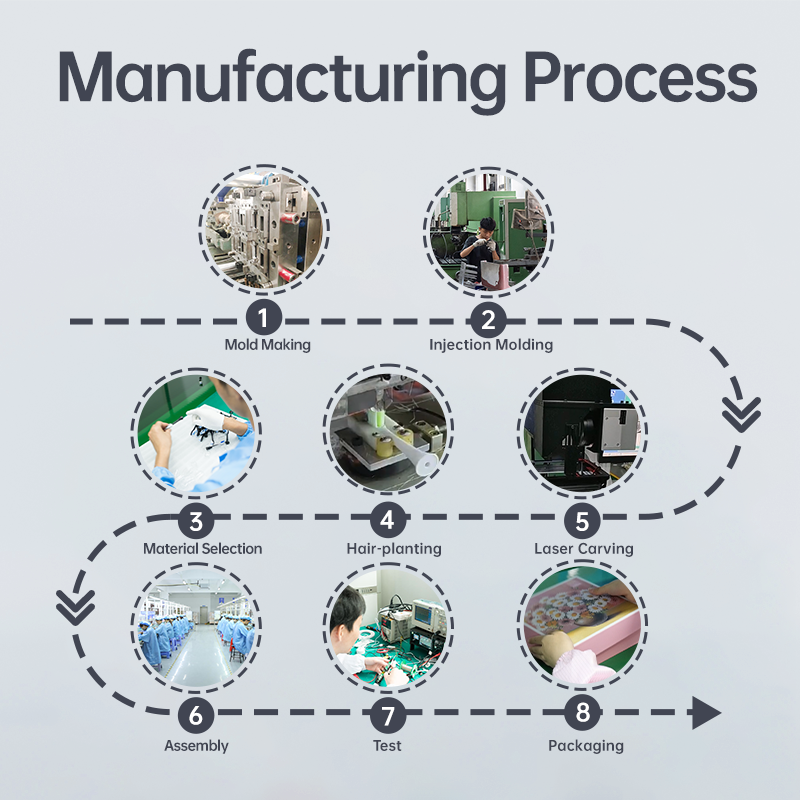
Does POWSMART Multi-Function Toothbrush Cause User Adaptation Struggles?
.jpg)
Custom Children’s Electric Toothbrush Design – Soft Bristles & Food-Grade Silicone
Gum Bleeding from Enamel Scratches – Time to Panic?

electric toothbrush factory evaluation standards- 5 Must-Read Criteria for Brand Owners

All-in-One Family Toothbrush Solution
1-scaled.jpg)
How Can Brand Owners Partner with Reliable Teeth Whitening Device Factories to Create Differentiated Products?
Allergic Reactions and Taste Alteration – Whitening’s Hidden Cost?
Tray Discomfort Triggering Mouth Ulcers – Coincidence?

How Can Oral Care Brands Enhance User Confidence Through Product Upgrades and Expansion?
Gel Leakage Causing Chemical Burns – Still Ignoring It?

Customization Teeth Whitening Gel

electric toothbrush heads Deep Clean

Private Label Whitening Gel
.jpg)
Florida Electric Toothbrush – Powsmart PTR-C8

electric toothbrush heads Ultra Soft

Electric toothbrush heads Charcoal Infused-Diamond

electric toothbrush heads Regular Clean

electric toothbrush heads Charcoal Infuse-Round
whstapp
whstapp
National Toll-Free Service Hotline
+86 755 86238638