For B2B product teams and OEM/ODM partners, the words Medical professional brush and Surgical grade toothbrush are sometimes used interchangeably — but they point to very different engineering, regulatory and commercial requirements. Below I unpack six clear dimensions so you can specify the right product for clinics, hospitals or retail-medical channels and avoid costly rework.
First and foremost, start with purpose. A Medical professional brush designed for routine clinical oral care: hygiene checks, patient home-care instruction, or chairside cleaning by dental staff. By contrast, a Surgical grade toothbrush is intended for peri-operative or high-acuity environments where minimising microbial loads (pre-op mouth prep, instrumentation suites) and sterility/traceability are essential.
Therefore:
Next, materials differ by risk and lifecycle. For a Medical professional brush you typically see durable, cleanable polymers and corrosion-resistant metals suitable for frequent disinfection. For a Surgical grade toothbrush materials and surface chemistry are chosen to be sterilizable and non-reactive:
In short, surgical grade equals higher material scrutiny and traceable certificates. Company web: https://www.powsmart.com/product/electric-toothbrush/
Crucially, the Surgical grade toothbrush must meet sterility workflows. That drives process and cost:
By contrast, a Medical professional brush may ship non-sterile with cleaning/disinfection instructions and a validated reprocessing SOP for clinic reuse.
Regulatory pathways separate the two categories:
Therefore, early engagement with regulatory and QA is mandatory when you intend to supply surgical grade items to hospitals.
Design choices follow the lifecycle model:
Commercial teams must model total cost of ownership (per patient use) and environmental impact when proposing either route.
Finally, what you prove drives adoption:
Hospitals and clinics will evaluate both specs and operational fit — not just “looks nice.”
If you’re deciding which product to build or certify, use this checklist:
Bottom line: a Medical professional brush engineered for clinical hygiene and frequent use under standard disinfection regimes; a Surgical grade toothbrush is a higher-risk product that must meet sterility, material and regulatory standards for use in sterile or peri-operative environments. Treat them as separate product families — not mere finish or branding variants — to avoid compliance gaps and ensure clinical adoption.
.jpg)
.jpg)
.jpg)
The Suitable Age for Children to Use Electric Toothbrushes – Factory’s View
-2-scaled.png)
The Scientific Principle of Red and Blue Light Teeth Whitening Device: How Can 460nm Blue Light and 630nm Red Light Safely Whiten Teeth?

How to Identify the Waterproof Rating of an Electric Toothbrush?

Why Do Some Electric Toothbrush Make a Lot of Noise?

How Can Dental Clinic Partnerships Promote Your Professional Whitening Kit?
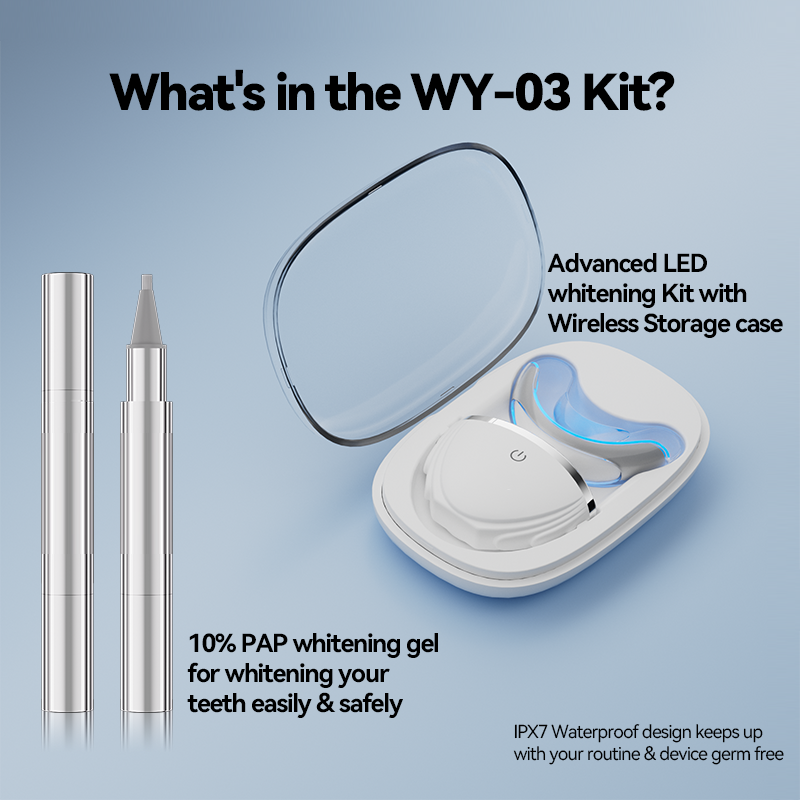
What Gives Some Teeth Whitening Devices Competitive advantage?

How to Evaluate an Electric Toothbrush Factory’s Comprehensive Capabilities

Analyze the Future Development Trend of the Oral Irrigator Market
.jpg)
Tampa Charger Replacement: Reliable Factory-Supplied Power Solutions
.jpg)
Professional Sonic Toothbrush OEM
Why Opt for Private Label Toothbrush Solutions in Handling Bulk Toothbrush Orders Efficiently?
.jpg)
Electric Toothbrush Couples Sharing Tips: Hygienic Practices for Two

How Can Water Flosser Factories Help Brand Owners Build High-End Product Lines?
.jpg)
Pump Failure Plus Tank Leakage – Terminal Malfunction or Preventable Issue?

How Does a Toothbrush Packaging Supplier Enable Custom Logo Packaging for Brand Identity?
.jpg)
How Does Water Flosser Prototype Development Benefit from Water Flosser Technology Partners?