In the competitive oral care industry, ensuring compliance with international safety standards is not optional—it’s essential. For manufacturers aiming to enter the U.S. market, teeth whitening device FDA certification is a key milestone. However, many products fail to pass FDA evaluation, delaying launches and damaging reputations. As a professional teeth whitening device factory, we have identified the three most common testing areas where home teeth whitening devices are rejected. This blog will walk through these tests and offer guidance on how manufacturers can avoid common pitfalls in the certification and safety of teeth whitening devices.
What is tested?
This test checks if any materials in the whitening device or the teeth whitening gel formulation cause irritation, toxicity, or allergic reactions when used in the mouth.
Why it leads to rejection:
If materials in the mouthpiece, LED casing, or especially the teeth whitening gel are not proven biocompatible, the FDA will reject the device. Non-medical-grade plastics or additives in gel formulas often trigger failures.
How to avoid failure:
Use FDA-registered, medical-grade silicone and plastics.
Ensure your teeth whitening gel supplier provides ISO 10993 and USP Class VI test reports.
Conduct biocompatibility testing early in the design phase.
Electrical devices must pass IEC 60601-1 or equivalent safety standards to ensure they do not pose a fire, shock, or electromagnetic interference risk during normal or abnormal use.
Why it leads to rejection:
Devices that overheat, lack proper insulation, or interfere with other electronics will not pass this test. Poor circuit board design or cheap components can cause serious compliance failures.
How to avoid failure:
Partner with experienced electronic engineers during product development.
Choose a teeth whitening device factory that already produces FDA-compliant models.
Pre-test prototypes with certified labs before full production.
The FDA closely inspects the product’s packaging, website content, and instructions. Any misleading claims about the device’s function, results, or status may result in immediate rejection
Why it leads to rejection:
Non-compliant labeling is one of the most common reasons for denial. Using vague or unsubstantiated performance claims—such as “whitens teeth in 5 minutes” or “safe for children”—without clinical backing violates FDA policy.
How to avoid failure:
Work with a regulatory consultant or compliance expert.
Avoid overpromising results in packaging or digital ads.
Use proper terminology: say “FDA-registered” instead of “FDA-approved” unless officially cleared through premarket approval (PMA) or 510(k).
Even if the product passes all tests, lacking proper documentation in your QMS (Quality Management System) can trigger rejection. FDA requires manufacturers to maintain detailed records for traceability, risk assessment, and corrective action.
Incorrect pH levels or over-limit hydrogen peroxide concentrations (typically >6% for OTC) can cause rejection or reclassification as a prescription-only product.
To avoid FDA rejection, manufacturers must go beyond basic product design. Compliance must be baked into every layer—from gel formulation to electrical safety to packaging language.
Here’s a quick success checklist for B2B buyers and OEM clients:
Choose a home teeth whitening devices factory with FDA experience
Use certified materials and components
Ensure home teeth whitening devices FDA certification supported by proper documentation
Verify the teeth whitening gel complies with both cosmetic and medical device guidelines
Build a compliant QMS from the beginning
Looking for an OEM/ODM partner with proven FDA compliance experience? Contact us to discuss compliant product development and regulatory support. https://www.powsmart.com/

Manufacturing Process and Quality Control for Electric Toothbrushes

Do LED teeth whitening device trays need to be removed and cleaned frequently?
.jpg)
Modern Lifestyle: Contemporary Electric Toothbrush OEM Designs
.jpg)
Water Flosser competitive Features That Cause Premium Pricing: What Brands Should Look For in Manufacturing
.jpg)
Need a toothbrush manufacturer China that specializes in kids electric toothbrush?
.jpg)
Tooth Microfractures Alongside Mucosal Abrasions – Urgent?
Subscription Model for Oral Care: OEM Strategies for Brush Head Replacement
How Do Hydro-dynamic Bearings Optimize Flow Channel Geometry in Water Flossers?
.jpg)
Navigating Electric Toothbrush Import Regulations?
.jpg)
dentist-approved sonic toothbrush factory
.jpg)
Long Battery Life Electric Toothbrush Wholesale – Bulk Supply for Retailers & Brands

How to Build a Successful Water Flosser Brand?

Why Measure Whitening Results Using the CIE Whiteness Index and Hue Saturation Lightness Metrics?
.jpg)
How Dangerous Are Jet Instability and Power Surges?

Can a Nozzle Storage Compartment Include a Jet Tip Sterilization Case for Hygiene?
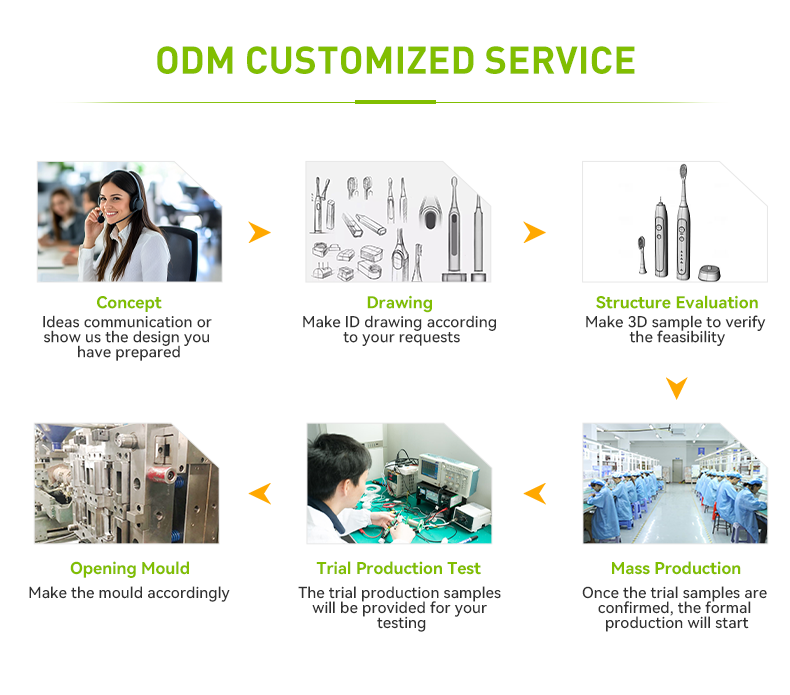
Why Combine Quality Certification Support with a Global Logistics Partner for International Markets?