In recent user surveys involving oral care devices, particularly toothbrushes and whitening tools, one recurring complaint has drawn attention: tongue irritation after repeated use. Upon deeper investigation, one subtle but critical factor stands out—cleaning residue. Whether from manufacturing, post-assembly processes, or insufficient end-product flushing, leftover surfactants or particulate contaminants may be causing discomfort in the most sensitive areas of the mouth. So, is this issue preventable? And what can manufacturers do to eliminate the risk at the source?
Cleaning residue refers to traces of chemical agents, particles, or detergent-based solutions that remain on product surfaces after manufacturing. These are often the result of:
For oral care products, especially those in direct contact with mucosal tissue (e.g. brush heads, whitening mouthpieces, tongue scrapers), even trace residue can become problematic.
The tongue contains thousands of exposed taste buds and nerve endings. It’s also in frequent motion and contact with oral care devices, making it especially susceptible to irritants. When cleaning residue remains on device surfaces:
What’s concerning is that many of these reactions occur gradually—meaning customers may not immediately associate them with the product. Company web:https://www.powsmart.com/product/electric-toothbrush/
A comprehensive defect analysis shows that cleaning residue typically stems from:
These oversights may not be detected in routine batch tests but will manifest through consumer experience—especially in export batches where climatic and regulatory conditions vary.
To avoid tongue irritation caused by cleaning residue, manufacturers must implement multi-stage controls:
For products involving silicone or TPU in contact with the tongue, baking-off volatile compounds (VOC) at high temperatures is also recommended pre-packaging.
B2B buyers now demand more than just verbal assurances. Manufacturers should:
These efforts help reduce the long-term cost of recalls and warranty claims caused by tongue irritation symptoms.
Instead of viewing residue control as a passive obligation, forward-thinking brands can market it as a trust-building feature:
In a saturated market, tongue safety and cleaning residue transparency can become a true differentiator—especially for sensitive-care or pediatric product lines.
Though easy to overlook, cleaning residue is a real and preventable cause of tongue irritation. It is not merely a hygiene issue but a product integrity challenge. B2B manufacturers that build robust, transparent cleaning validation into their production line will not only avoid user complaints but stand out as leaders in safety-focused, medically conscious device manufacturing. Contact us
.jpg)
.jpg)
.jpg)
Is Your Water Flosser Plagued by Nozzle Clogging and Water Tank Leakage?

How Does This Quiet Electric Toothbrush Score in Noise Comparison Test?
.jpg)
Whitening & Polishing Electric Toothbrush – OEM/ODM Services
Why Opt for Private Label Toothbrush Solutions in Handling Bulk Toothbrush Orders Efficiently?
.jpg)
dental whitening device ODM project | Custom LED Whitening Device Development

Is a TSA Friendly Toothbrush with Portable Toothbrush Case Truly Hassle-Free at Airports?

Adventure-Ready Electric Toothbrushes: Outdoor Toothbrush OEM Features for Outdoor Enthusiasts
.jpg)
Hose Cracks Causing Water Pressure Instability?

Which Teeth Whitening Method Is Right for You?

Does Dental Grade Whitening Necessitate the Use of Safe Whitening Ingredients?
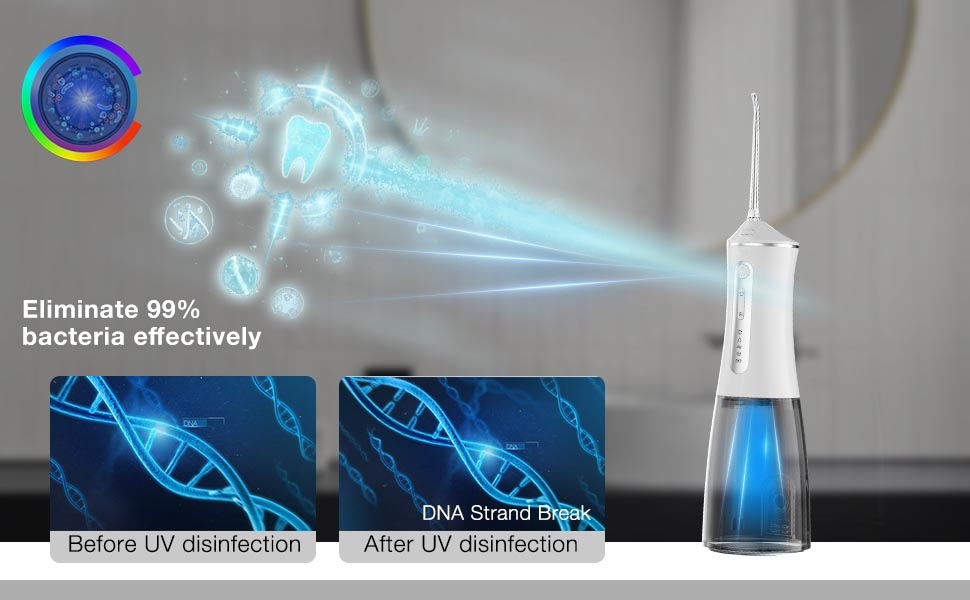
How Does a Polishing Brush Head Enable Enamel-Safe Whitening?
How to Repair an Electric Toothbrush – Answers from Manufacturers
.jpg)
Private Label Dental Products China | OEM and Bulk Supply
.jpg)
Vestibular Stimulation Worsening Tooth Demineralization?
.jpg)
High-Frequency Toothbrush Wholesale for Global B2B Distribution
.jpg)
Boston Electric Toothbrush for Sensitive Gums: Gentle Yet Powerful